Summary
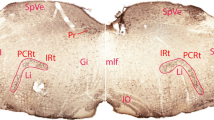
Hypothalamic efferents to the lateral reticular nucleus (NRL) have been demonstrated in the cat by means of anterograde and retrograde axonal transport of the wheat germ agglutinin — horseradish peroxidase (WGA-HRP) complex. Pressure injections of the WGA-HRP complex into the hypothalamus resulted in anterograde labelling of branching terminal axons both in the NRL and in an adjacent area, presumably the ventrolateral catecholaminergic cell group (A1). After microiontophoretical ejections of the WGA-HRP complex into the NRL from a ventral approach, retrogradely labelled neurons were found in the lateral, dorsal, posterior and anterior hypothalamic areas and in the tubero-mammillary, dorsomedial and periventricular nuclei. The projection is bilateral with a clear ipsilateral predominance and has its main origin in the lateral hypothalamic area. The locations of hypothalamic cells projecting to the NRL are somewhat different from those giving rise to hypothalamo-cerebellar and hypothalamo-spinal connections. The present demonstration of a hypothalamic input to one of the major precerebellar relay nuclei introduces a new possible indirect route through which the cerebellum may be influenced by the hypothalamus. The different indirect and direct hypothalamo-cerebellar pathways and their potential functional importance are discussed.
Similar content being viewed by others
References
Andrezik JA, Dormer KJ, Foreman RD, Person RJ (1984) Fastigial nucleus projections to the brain stem in beagles: pathways for autonomic regulation. Neuroscience 11: 497–507
Ban T (1964) The hypothalamus, especially on its fiber connections and the septo-preoptico-hypothalamic system. Med J Osaka Univ 15: 58–83
Basbaum AI, Fields HL (1979) The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol 187: 513–532
Beattie J, Brow GR, Long CNH (1930) Physiological and anatomical evidence for the existence of nerve tracts connecting the hypothalamus with spinal sympathetic centres. Proc R Soc B 106: 253–275
Berman AJ, Berman D, Prescott JW (1974) The effect of cerebellar lesions on emotional behavior in the rhesus monkey. In: Cooper IS, Riklan M, Snider RS (eds) The cerebellum, epilepsy, and behavior. Plenum Press, New York, pp 277–284
Bleier R (1961) The hypothalamus of the cat. The Johns Hopkins Press, Baltimore, MD
Blessing WW, Frost P, Furness JB (1980) Catecholamine cell groups of the cat medulla oblongata. Brain Res 192: 69–75
Brodal A (1943) The cerebellar connections of the nucleus reticularis lateralis (nucleus funiculi lateralis) in rabbit and cat. Acta Psychiat 18: 171–233
Brodal P (1975) Demonstration of a somatotopically organized projection onto the paramedian lobule and the anterior lobe from the lateral reticular nucleus: an experimental study with the horseradish peroxidase method. Brain Res 95: 221–239
Brodal P, Dietrichs E, Bjaalie JG, Nordby T, Walberg F (1983) Is lectin-coupled horseradish peroxidase taken up and transported by undamaged as well as damaged fibers in the central nervous system? Brain Res 278: 1–9
Carpenter MB, Batton RR III (1982) Connections of the fastigial nucleus in the cat and monkey. Exp Brain Res Suppl 6: 250–291
Chambers WW (1947) Electrical stimulation of the interior of the cerebellum in the cat. Am J Anat 80: 55–93
Cohen D, Chambers WW, Sprague JM (1958) Experimental study of the efferent projections from the cerebellar nuclei to the brainstem of the cat. J Comp Neurol 109: 233–259
Cooper IS, Amin I, Riklan M, Waltz JM, Poon TP (1976) Chronic cerebellar stimulation in epilepsy. Arch Neurol 33: 559–570
Dahlström A, Fuxe K (1964) Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand 62: Suppl 232: 1–55
Dietrichs E (1983a) Cerebellar cortical afferents from the periaqueductal grey in the cat. Neurosci Lett 41: 21–26
Dietrichs E (1983b) Cerebellar nuclear afferents from the lateral reticular nucleus in the cat. Brain Res 288: 320–324
Dietrichs E (1984) Cerebellar autonomic function: Direct hypothalamo-cerebellar pathway. Science 223: 591–593
Dietrichs E, Haines DE (1984) Demonstration of hypothalamocerebellar and cerebello-hypothalamic fibres in a prosimian primate (Galago crassicaudatus). Anat Embryol 170: 313–318
Dietrichs E, Haines DE (1985a) Observations on the cerebello-Hypothalmic projection, with comments on non-somatic cerebellar circuits. Arch Ital Biol (in press)
Dietrichs E, Haines DE (1985b) Do hypothalamo-cerebellar fibres terminate in all layers of the cerebellar cortex? Anat. Embryol. (in press)
Dietrichs E, Walberg F (1979) The cerebellar projection from the lateral reticular nucleus as studied with retrograde transport of horseradish peroxidase. Anat Embryol 155: 273–290
Dietrichs E, Zheng Z-H (1984) Are hypothalamo-cerebellar fibers collaterals from the hypothalamo-spinal projection? Brain Res 296: 225–231
Dietrichs, E, Walberg F, Haines DE (1985) Cerebellar nuclear afferents from feline hypothalamus demonstrated by retrograde transport after implantation of crystalline WGA-HRP. Neurosci Lett 54: 129–133
Dow RS (1974) Some novel concepts of cerebellar physiology. Mount Sinai J Med 61: 103–119
Dow RS, Moruzzi G (1958) The physiology and pathology of the cerebellum. Univ Minnesota Press, Minneapolis
Gerben MJ (1969) Control of treadmill exercise of rats with hypothalamic stimulation. Behav Res Meth Instrum 1: 309–311
Gladfelter WE, Brobeck JR (1962) Decreased spontaneous locomotor activity in the rat induced by hypothalamic lesions. Am J Physiol 203: 811–817
Gonatas NK, Harper C, Mizutani T, Gonatas JO (1979) Superior sensitivity of conjugates of horseradish peroxidase with wheat germ agglutinin for studies of retrograde axonal transport. J Histochem Cytochem 27: 728–734
Haines DE, Dietrichs E (1984) An HRP study of hypothalamocerebellar and cerebello-hypothalamic connections in squirrel monkey (Saimiri sciureus). J Comp Neurol 229: 559–575
Haines DE, Dietrichs E, Sowa TE (1984) Hypothalamo-cerebellar and cerebello-hypothalamic pathways: a review and hypothesis concerning cerebellar circuits which may influence autonomic centers and affective behaviour. Brain Behav Evol 24: 198–220
Haines DE, Sowa TE, Dietrichs E (1985) Connections between the cerebellum and hypothalamus in the tree shrew (Tupaiaglis) Brain Res 328: 367–373
Hancock MB (1976) Cells of origin of hypothalamo-spinal projections in the rat. Neurosci Lett 3: 179–184
Hosoya Y, Matsushita M (1979) Identification and distribution of the spinal and hypophyseal projection neurons in the paraventricular nucleus of the rat. A light and electron microscopic study with the horseradish peroxidase method. Exp Brain Res 35: 315–331
Jacobs VL (1965) The cerebellofugal system in the tarsius (Tarsiidae carbonarius) and the marmoset (Oedipomidas oedipus). (Thesis) Univ Kansas
Jones BE, Friedman L (1983) Atlas of catecholamine perikarya, varicosities and pathways in the brainstem of the cat. J Comp Neurol 215: 382–396
Kneisley LW, Biber MP, LaVail JH (1978) A study of the origin of brain stem projections to monkey spinal cord using the retrograde transport method. Exp Neurol 60: 116–139
Künzle H (1975) Autoradiographic tracing of the cerebellar projections from the lateral reticular nucleus in the cat. Exp Brain Res 22: 255–266
Kuypers HGJM, Maisky VA (1975) Retrograde axonal transport of horseradish peroxidase from spinal cord to brain stem cell groups in the cat. Neurosci Lett 1: 9–14
Martin GF, King JS, Dom R (1974) The projections to the deep cerebellar nuclei of the opossum, Didelphis marsupialis virginiana. J Hirnforsch 15: 545–573
Matsushita M, Ikeda M (1976) Projections from the lateral reticular nucleus to the cerebellar cortex and nuclei in the cat. Exp Brain Res 24: 403–421
Mesulam M-M (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106–117
Miller AD, Wilson VJ (1983) Vestibular-induced vomiting after vestibulocerebellar lesions. Brain Behav Evol 23: 26–31
Moruzzi G (1940) Paleocerebellar inhibition of vasomotor and respiratory carotid sinus reflexes. J Neurophysiol 3: 20–31
Moruzzi G (1950) Problems in cerebellar physiology. C.C. Thomas, Springfield Ill
Olson L, Fuxe K (1972) Further mapping out of central nor-adrenaline neuron systems: projections of the ‘subcoeruleus’ area. Brain Res 43: 289–295
Orlovskii GN (1969) Spontaneous and induced locomotion of the thalamic cat. Biophysics 14: 1154–1162
Palkovits M, Záborszky L, Feminger A, Mezey É, Fekete MIK, Herman JP, Kanyicska B, Szabó D (1980) Noradrenergic innervation of the rat hypothalamus: experimental biochemical and electron microscopic studies. Brain Res 191: 161–171
Poitras D, Parent A (1978) Atlas of the distribution of monoamine-containing nerve cell bodies in the brain stem of the cat. J Comp Neurol 179: 699–718
Rosenquist AC, Hoebel BG (1968) Wheel running elicited by electrical stimulation of the brain. Physiol Behav 3: 563–566
Sakumoto T, Tohyama M, Satoh K, Kimoto Y, Kinugasa T, Tanizawa O, Kurachi K, Shimizu N (1978) Afferent fiber connections from lower brain stem to hypothalamus studied by the horseradish peroxidase method with special reference to noradrenaline innervation. Exp Brain Res 31: 81–94
Saper CB, Loewy AD, Swanson LW, Cowan WM (1976) Direct hypothalamo-autonomic connections. Brain Res 117: 305–312
Sawchenko PE, Swanson LW (1982) The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res Rev 4: 275–325
Sawyer DH, Hilliard J, Ban T (1961) Autonomic and EEG responses to cerebellar stimulation in rabbits. Am J Physiol 200: 405–412
Somana E, Walberg F (1979) Cerebellar afferents from the nucleus of the solitary tract. Neurosci Lett 11: 41–47
Swanson LW, Kuypers HGJM (1980) The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570
van Buren JM, Wood JH, Oakley J, Hambrecht F (1978) Preliminary evaluation of cerebellar stimulation by double-blind stimulation and biological criteria in the treatment of epilepsy. J Neurosurg 48: 407–416
Walberg F (1952) The lateral reticular nucleus of the medulla oblongata in mammals. J Comp Neurol 96: 283–343
Walberg F (1981) The diencephalo-olivary projection in the cat as studied with retrograde transport of horseradish peroxidase. Anat Embryol 163: 223–234
Walberg F (1982a) The vestibule- and reticulocerebellar projections in the cat as studied with horseradish peroxidase as a retrograde tracer. In: Palay SL, Chan-Palay V (eds) The cerebellum — new vistas. Exp Brain Res, Suppl 6: 477–498
Walberg F (1982b) Paths descending from the brain stem — an overview. In: Sjölund B, Björklund A (eds) Brain stem control of spinal mechanisms. Elsevier Biomedical Press, pp 1–27
Wallenberg A (1905) Sekundäre Bahnen aus den frontalen sensiblen Trigeminuskernen des Kaninchens. Anat Anz 26: 145–155
Wayner MJ, Barone FC, Loullis CC (1981) The lateral hypothalamus and adjunctive behavior. In: Morgane PS, Panksepp J (eds) Handbook of the hypothalamus. Vol 3, Part B: Behavioral studies of the hypothalamus. Marcel Dekker Inc, New York, pp 107–145
Wiklund L, Léger L, Persson M (1981) Monoamine cell distribution in the cat brain stem. A fluorescence histochemical study with quantification of indolaminergic and locus coeruleus cell groups. J Comp Neurol 203: 613–647
Zanchetti A, Zoccolini A (1954) Autonomic hypothalamic outbursts elicited by cerebellar stimulation. J Neurophysiol 17: 475–483
Zheng Z-H, Dietrichs E, Walberg F (1982) Cerebellar afferent fibres from the dorsal motor vagal nucleus in the cat. Neurosci Lett 32: 113–118
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dietrichs, E., Haines, D.E. & Qvist, H. Indirect hypothalamo-cerebellar pathway? Demonstration of hypothalamic efferents to the lateral reticular nucleus. Exp Brain Res 60, 483–491 (1985). https://doi.org/10.1007/BF00236933
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00236933