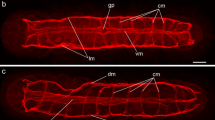
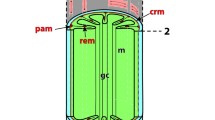
Summary
The morphology of tissue channels in muscle and neural tissues of Octopus was investigated, at the ultrastructural level, with a technique involving the precipitation of ferrocyanide ions. The numbers, sizes and conductivities of the channels were estimated from quantitative data. No evidence was gained to indicate that the low microvascular density in Octopus is coupled to an especially extensive network of extravascular channels. The tissue channel system in Octopus appears to be broadly comparable with the mammalian system; a lack of information prevents more appropriate comparisons with marine fishes. Probable functions of tissue channels in Octopus and mammals, and reasons for apparent similarities and differences in the channel organization of these divergent groups, are discussed.
Similar content being viewed by others
References
Altman PL, Dittmer DS (1971) “Respiration and Circulation”. Federation of American Societies for Experimental Biology
Barber VC, Graziadei P (1965) The fine structure of cephalopod blood vessels. I. Some smaller peripheral vessels. Z Zellforsch 66:765–781
Barber VC, Graziadei P (1967) The fine structure of cephalopod blood vessels. II. The vessels of the nervous system. Z Zellforsch 77:147–161
Berry CF, Cottrell GA (1970) Neurosecretion in the vena cava of the cephalopod Eledone cirrosa. Z Zellforsch 104:107–115
Browning J (1979) Octopus microvasculature: permeability to ferritin and carbon. Tissue Cell 11:371–383
Browning J (1980) Demarcation of tissue channels by ferrocyanide deposits: use of an alternative precipitant. Microvasc Res 19:380–384
Browning J, Casley-Smith JR (1979) Fine structural studies of the permeability of Octopus microvasculature to macromolecules. Bibl Anat 18:38–40
Casley-Smith JR (1980a) Comparative fine structure of the microvasculature. In: Altura BM (eds) Vascular Endothelium and Basement Membranes. Karger, Basel NY, Advances in Microcirculation 9:1–44
Casley-Smith JR (1980b) The structure and functioning of the blood vessels, interstitial tissues, and lymphatics. In: Földi M (eds) Lymphology. Thieme, Stuttgart (in press)
Casley-Smith JR, Vincent AH (1978) The quantitative morphology of interstitial channels in some tissues of the rat and rabbit. Tissue Cell 10:571–584
Casley-Smith JR, O'Donoghue PJO, Crocker KWJ (1975) The quantitative relationships between fenestrae in jejunal capillaries and connective tissue channels: Proof of “tunnel capillaries”. Microvasc Res 9:78–100
Casley-Smith JR, Földi-Börcsök E, Földi M (1979) A fine structural study of the tissue channels' numbers and dimensions in normal and lymphoedematous tissues. Z Lymphol 3:49–58
Chase WH (1959) Extracellular distribution of ferrocyanide in muscle. Arch Pathol 67:525–532
Dennis JB (1959) Effects of various factors on the distribution of ferrocyanide in ground substance. Arch Pathol 67:533–549
Diana JN, Fleming BP (1979) Some current problems in microvascular research. Microvasc Res 18:144–152
Dykens JA, Mangum CP (1979) The design of cardiac muscle and the mode of metabolism in molluscs. Comp Biochem Physiol 62:549–554
Froesch D (1974) The subpedunculate lobe of the octopus brain: Evidence for dual function. Brain Res 75:277–285
Froesch D, Mangold K (1976) On the structure and function of a neurohemal organ in the eye cavity of Elodone cirrosa (Cephalopoda). Brain Res 111:287–293
Glauert AM (1975) Part I: Fixation, dehydration and embedding of biological specimens. In: Practical Methods in Electron Microscopy, Vol. III. North-Holland Publishing Co, Amsterdam
Gordon MS (1972) Animal Physiology: Principles and Adaptations. The Macmillan Co, London
Gray EG (1969) Electron microscopy of the glio-vascular organization of the brain of Octopus. Phil Trans R Soc Lond B, 255:13–32
Guyton AC, Scheel K, Murphree D (1966) Interstitial fluid pressure: III. Its effects on resistance to tissue fluid mobility. Circ Res 19:412–419
Intaglietta M, Deplomb EP (1973) Fluid exchange in tunnel and tube capillaries. Microvasc Res, 6:153–168
Krogh A (1919) The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. J Physiol 52:391–408
Lasansky A, Wald F (1962) The extracellular space in the toad retina as defined by the distribution of ferrocyanide. A light and electron microscope study. J Cell Biol 15:463–479
Longmuir IS, Bourke A (1960) The measurement of the diffusion of oxygen through respiring tissue. Biochem J 76:225–229
Maginniss LA, Wells MJ (1969) The oxygen consumption of Octopus cyanea. J Exp Biol 51:607–613
Martin AW, Harrison FM, Huston MJ, Stewart DM (1958) The blood of some representative molluscs. J Exp Biol 35:260–279
Martin R (1968) Fine structure of the neurosecretory system of the vena cava in Octopus. Brain Res 8:201–205
McDougall JOB, McCabe M (1967) Diffusion coefficient of oxygen through tissues. Nature 215:1173–1174
Pritchard AW, Huston MJ, Martin AW (1963) Effects of in vitro anoxia on metabolism of Octopus heart tissue. Proc Soc Exp Biol Med 112:27–29
Rodbard S (1975) Selective staining of fibrous connective tissue capsules and lymphatics. An evaluation of “interstitial” fluids. Lymphology 8:142–148
Smith LS (1962) Some aspects of peripheral circulation in Octopus dofleini. Doctoral Dissertation, Univ Washington, Seattle, Washington
Smith LS (1963) Circulatory anatomy of the octopus arm. J Morphol 113:261–266
Weast RC (1975) Handbook of Chemistry and Physics, 56th edition. Chemical Rubber Publishing Co
Wells MJ (1978) Octopus. Chapman and Hall, London
Witte S (1975) Microscopic techniques for the in situ characterization of concentration of tissue components and penetrating molecules. Biorheology 12:173–180
Wolff JR (1977) Ultrastructure of the terminal vascular bed as related to function. In: Kaley G, Altura BM (eds) Microcirculation, Vol I, P 95–130
Young JZ (1971) Blood vessels and glia of the nervous system. In: “The Anatomy of the Nervous System of Octopus vulgaris”. Ch 20, Clarendon Press, Oxford
Young JZ (1976) The nervous system of Loligo. II. Suboesophageal centres. Phil Trans R Soc B 274:101–167
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Browning, J., Casley-Smith, J.R. Tissue channel morphology in Octopus . Cell Tissue Res. 215, 153–170 (1981). https://doi.org/10.1007/BF00236256
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00236256