Abstract
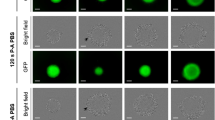
Permeabilization of L1210 cells by lithotripter shock waves in vitro was monitored by evaluating the accumulation of fluorescein-labeled dextrans with a relative molecular mass ranging from 3,900–2,000,000. Incubation with labeled dextran alone caused a dose- and time-dependent increase in cellular fluorescence as determined by flow cytometry, with a vesicular distribution pattern in the cells consistent with endocytotic uptake. Shock wave exposure prior to incubation with labeled dextran revealed similar fluorescence intensities compared to incubation with labeled dextran alone. When cells were exposed to shock waves in the presence of labeled dextran, mean cellular fluorescence was further increased, indicating additional internalization of the probe. Confocal laser scanning microscopy confirmed intracellular fluorescence of labeled dextran with a diffuse distribution pattern. Fluorescence-activated cell sorting with subsequent determination of proliferation revealed that permeabilized cells were viable and able to proliferate. Permeabilization of the membrane of L1210 cells by shock waves in vitro allowed loading of dextrans with a relative molecular mass up to 2,000,000.
Permeabilization of tumor cells by shock waves provides a useful tool for introducing molecules into cells which might be of interest for drug targeting in tumor therapy in vivo.
Similar content being viewed by others
References
Arbuthnott, J.P., Freer, J.H., Bernheimer, A.W. 1967. Physical states of staphylococcal α-toxin. J. Bacteriol. 94:1170–1177
Bangham, A.D., Horne, R.W. 1962. Action of saponin on biological cell membranes. Nature 196:952–953
Bartoletti, D.C., Harrison, G.I., Weaver, C. 1989. The number of molecules taken up by electroporated cells: quantitative determination. FEBS Lett. 256:4–10
Brümmer, F., Brenner, J., Bräuner, T., Hülser, D.F. 1989. Effect of shock waves on suspended and immobilized L1210 cells. Ultrasound Med. Biol. 15:229–239
Carmichael, J., Degraff, W.G., Gazdar, A.F., Minna, J.D., Mitchell, J.B. 1987. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 47:936–942
Castellot, J.J., Miller, M.R., Pardee, A.B. 1978. Animal cells reversibly permeable to small molecules. Proc. Natl. Acad. Sci. USA 75:351–355
Coleman, A., Saunders, J., Crum, L., Dyson, M. 1987. Pressure waveforms generated by a Dornier extracorporeal shock-wave lithotripter. Ultrasound Med. Biol. 13:69–76
Crum, L.A. 1982. Acoustic cavitation. Proc. 1982 IEEE Ultrasonics Symposium, NY: IEEE 1982:1–11
Dimitrov, D.S., Sowers, A.E. 1990. Membrane electroporation—fast molecular exchange by electroosmosis. Biochim. Biophys. Acta. 1022:381–392
Duncan, J.L. 1974. Characteristics of streptolysin O hemolysis: kinetics of hemoglobin and 86rubidium release. Infect. Immun. 9:1022–1027
Gambihler, S., Delius, M., Brendel, W. 1990. Biological effects of shock waves: Cell disruption, viability, and proliferation of L1210 cells exposed to shock waves in vitro. Ultrasound Med. Biol. 16:587–594
Gambihler, S., Delius, M., Ellwart, J.W. 1992. Transient increase in membrane permeability of L1210 cells upon exposure to lithotripter shock waves in vitro. Naturwissenschaften 79:328–329
Glogauer, M., McCulloch, C.A.G. 1992. Introduction of large molecules into viable fibroblasts by electroporation: optimization of loading and identification of labeled cellular compartments. Exp. Cell Res. 200:227–234
Hoffman, J.F. 1962. The active transport of sodium by ghosts of human red blood cells. J. Gen. Physiol. 45:837–859
Lambert, H., Pankov, R., Gauthier, J., Hannock, R. 1990. Electroporation-mediated uptake of proteins into mammalian cells. Biochem. Cell. Biol. 68:729–734
Lieber, M.R., Steck, T.L. 1982. A description of the holes in human erythrocyte membrane ghosts. J. Biol. Chem. 257:11651–11659
Lorimer, J.P. 1991. The effect of ultrasound on the degradation of aqueous native dextran. In: Ultrasonics International 91 Conference Proceedings. pp. 649–654. Butterworth-Heinemann, Oxford
McNeil, P.L. 1989. Incorporation of macromolecules into living cells. Methods Cell Biol. 29:153–173
McNeil, P.L., Murphy, R.F., Lanni, F., Taylor, D.L. 1984. A method for incorporating macromolecules into adherent cells. J. Cell Biol. 98:1556–1564
Michel, M.R., Elgizoli, M., Koblet, H., Kempf, C. 1988. Diffusion loading conditions determine recovery of protein synthesis in electroporated P3X63Ag8 cells. Experientia 44:199–203
Mueller, M. 1990. Comparison of Dornier lithotripters. Measurement of shock wave fields and fragmentation effectiveness. Biomed. Tech. 35:250–262
Oosterhof, G.O.N., Smits, G.A.H.J., de Ruyter, A.E., van Moorselaar, R.J.A., Schalken, J.A., Debruyne, F.M.J. 1989. The in vitro effect of electromagnetically generated shock waves (Lithostar) on the Dunning R3327 PAT-2 rat prostatic cancer cell-line. Urol. Res. 17:13–19
Pasternak, C.A., Micklem, K.J. 1973. Permeability changes during cell fusion. J. Membrane Biol. 14:293–303
Rozengurt, E., Heppel, L.A. 1975. A specific effect of external ATP on the permeability of transformed 3T3 cells. Biochem. Biophys. Res. Commun. 67:1581–1588
Russo, P., Stephenson, A., Mies, C., Huryk, R., Heston, W.D.W., Melamed, M.R., Fair, W.R. 1986. High energy shock waves suppress tumor growth in vitro and in vivo. J. Urol. 135:626–628
Stein, W.D. 1981. Permeability for lipophilic molecules. In: Membrane Transport. S.L. Bonting, J.J.H.H.M. de Pont, editors. pp. 1–28. Elsevier/North-Holland, Amsterdam
Steinbach, P., Hofstädter, F., Nicolai, H., Rössler, W., Wieland, W. 1992. In vitro investigations on cellular damage induced by high energy shock waves. Ultrasound Med. Biol. 18:691–699
Swanson, J. 1989. Fluorescent labeling of endocytic compartments. Methods Cell Biol. 29:137–151
Vogel, A., Lauterborn, E. 1988. Time-resolved particle image velocimetry used in the investigation of cavitation bubble dynamics. Appl. Opt. 27:1869–1876
Wilson, A.K., Horwitz, J., de Lanerolle, P. 1991. Evaluation of the electroinjection method for introducing proteins into living cells. Am. J. Physiol. 260:C355-C363
Zimmermann, U. 1986. Electrical breakdown, electropermeabilization and electrofusion. Rev. Physiol. Biochem. Pharmacol. 105: 175–256
Zimmermann, U., Schnettler, R., Klöck, H., Donath, E., Glaser, R.W. 1990. Mechanisms of electrostimulated uptake of macromolecules into living cells. Naturwissenschaften 77:543–545
Zuurendonk, P.F., Tager, J.M. 1974. Rapid separation of paniculate components and soluble cytoplasm of isolated rat-liver cells. Biochim. Biophys. Acta 333:393–399
Author information
Authors and Affiliations
Additional information
This work was supported by the Deutsche Forschungsgemeinschaft grant De 531/1-1. We are particularly grateful to Dr. Ulrich Dirnagl (Department of Neurology, University of Munich, Marchioninistr. 15, 81377 Munich, Germany) for performing the confocal laser scanning microscopy and to Gerhard Adams for excellent technical assistance.
Rights and permissions
About this article
Cite this article
Gambihler, S., Delhis, M. & Ellwart, J.W. Permeabilization of the plasma membrane of L1210 mouse leukemia cells using lithotripter shock waves. J. Membarin Biol. 141, 267–275 (1994). https://doi.org/10.1007/BF00235136
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00235136