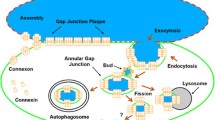
Abstract
The rapid effects of cAMP on gap junction-mediated intercellular communication were examined in several cell types which express different levels of the gap junction protein, connexin43 (Cx43), including immortalized rat hepatocyte and granulosa cells, bovine coronary venular endothelial cells, primary rat myometrial and equine uterine epithelial cells. Functional analysis of changes in junctional communication induced by 8-bromo-cAMP was monitored by a fluorescence recovery after photobleaching assay in subconfluent cultures in the presence or absence of 1.0 mm 1-octanol (an agent which uncouples cells by closing gap junction channels). Communicating cells treated with 1.0 mm 8-bromo-cAMP alone exhibited significant increases in the percent of fluorescence recovery which were detected within 1–3 min depending on cell type, and junctional communication remained significantly elevated for up to 24 hr. Addition of 1.0 mm 8-bromo-cAMP to cultured cells, which were uncoupled with 1.0 mm octanol for 1 min, exhibited partial restoration of gap junctional permeability beginning within 3–5 min. Identical treatments were performed on cultures that were subsequently processed for indirect immunofluorescence to monitor Cx43 distribution. The changes in junctional permeability of cells correlated with changes in the distribution of immunoreactive Cx43. Cells treated for 2 hr with 10 μm monensin exhibited a reduced communication rate which was accompanied by increased vesicular cytoplasmic Cx43 staining and reduced punctate surface staining of junctional plaques. Addition of 1.0 mm 8-bromo-cAMP to these cultures had no effect on the rate of communication or the distribution of Cx43 compared to cultures treated with monensin alone. These data suggest that an effect of cyclic AMP on Cx43 gap junctions is to promote increases in gap junctional permeability by increasing trafficking and/or assembly of Cx43 to plasma membrane gap junctional plaques.
Similar content being viewed by others
References
Barhoumi, R., Bowen, J.A., Stein, L.S., Echols, J., Burghardt, R.C. 1993. Concurrent analysis of intracellular gluthathione content and gap junctional intercellular communication. Cytometry 14:747–756
Berthoud, V.M., Ledbetter, M.L.S., Hertzberg, E.L., Saez, J.C. 1992. Connexin43 in MDCK cells: regulation by a tumor-promoting phorbol ester and Ca2+. Eur. J. Cell Biol. 57:40–50
Brady, H.A., Burghardt, R.C., Evans, J.W., Blanchard, T.L., Varner, D.D., Bruemmer, J.E. 1993. Model system for the study of uterine/ trophoblast interactions in the mare. J Equine Vet. Sci. 13:506–511
Brissette, J.L., Kumar, N.M., Gilula, N.B., Dotto, G.P. 1991. The tumor promoter 12-O-tetradecanoylphorbol-13-acetate and the ras oncogene modulate expression and phosphorylation of gap junction proteins. Mol. Cell. Biol. 11:5364–5371
Burghardt, R.C., Barhoumi, R., Dookwah, H.D. 1993. Endocrine control of myometrial gap junctions and their control during parturition. Semin. Reprod. Endocrinol. 11:250–260
Burghardt, R.C., Barhoumi, R., Doolittle, D., Phillips, T.D. 1994. Application of Cellular Fluorescence Imaging for In Vitro Toxicology Testing. In: Principles and Methods of Toxicology, 3rd Edition, A.W. Hayes, editor, pp. 1231–1259. Raven Press, New York
Burt, J.M. 1991. Modulation of cardiac gap junction channel activity by the membrane lipid environment. In: Biophysics of Gap Junction Channels. C. Peracchia, editor, pp. 75–93. CRC Press, Boca Raton
Chanson, M., Bruzzone, R., Bosco, D., Meda, P. 1989. Effects of n-alcohols on junctional coupling and amylase secretion of pancreatic acinar cells. J. Cell. Physiol. 139:147–156
Cole, W.C., Garfield, R.E. 1986. Evidence for physiological regulation of myometrial gap junction permeability. Am. J. Physiol. 251:C411-C420
Crow, D.S., Beyer, E.C., Paul, D.L., Kobe, S.S., Lau, A.F. 1990. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol. Cell. Biol. 10:1754–1763
De Mello, W.C. 1994. Gap junctional communication in excitable tissues; the heart as a paradigma. Prog. Biophys. Molec. Biol. 61:1–35
Dookwah, H.D., Barhoumi, R., Narasimhan, T.K., Safe, S.H., Burghardt, R.C. 1992. Gap junctions in myometrial cell cultures: evidence for modulation by cAMP. Biol. Reprod. 47:397–406
Filson, A.J., Azarnia, R., Beyer, E.C., Loewenstein, W.R., Brugge, J.S. 1990. Tyrosine phosphorylation correlates with inhibition of cell-to-cell communication. Cell Growth and Differentiation 1:661–668
Flagg-Newton, J.L., Dahl, G., Loewenstein, W.R. 1981. Cell junction and cyclic AMP: I. Upregulation of junctional membrane permeability and junctional membrane particles by administration of cyclic nucleotide or phosphodiesterase inhibitor. J. Membrane Biol. 63:105–121
Godwin, A.J., Green, L.M., Walsh, M.P., McDonald, J.R., Walsh, D.A., Fletcher, W.H. 1993. In situ regulation of cell-cell communication by the cAMP-dependent protein kinase and protein kinase C. Mol. Cell. Biochem. 127/128:293–307
Goldberg, G.S., Lau, A.F. 1993. Dynamics of connexin43 phosphorylation in pp60v-src-transformed cells. Biochem. J. 295:735–742
Granot, I., Dekel, N. 1994. Phosphorylation and expression of connexin-43 ovarian gap junction protein are regulated by luteinizing hormone. J. Biol. Chem. 48:30502–30509
Hall, J.E., Zampighi, G.A., Davis, R.M. 1993. Progress in Cell Research, Volume 3. Gap Junctions. Elsevier Science Publishers, Amsterdam
Hii, C.S.T., Schmidt, S.A., Clark, K.J., Murray, A.W. 1994. Lysophosphatidic acid inhibits gap-junctional communication and stimulates phosphorylation of connexin-43 in WB cells: possible involvement of the mitogen-activated protein kinase cascade. Biochem. J. 303:475–479
Kanemitsu, M.Y., Lau, A.F. 1993. Epidermal growth factor stimulates the disruption of gap junctional communication and connexin43 phosphorylation independent of 12-O-tetradecanoylphorbol 12-acetate-sensitive protein kinase C: the possible involvement of mitogen-activated protein kinase. Molec. Biol. Cell 4:837–848
Kurata, W.E., Lau, A.F. 1994. p130gag-fps disrupts gap junctional communication and induces phosphorylation of connexin43 in a manner similar to that of pp60v-src. Oncogene 9:329–335
Little, T.L., Beyer, E.C., Duling, B.R. 1995. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am. J. Physiol. 268:H729-H737
Loewenstein, W.R. 1985. Regulation of cell-to-cell communication by phosphorylation. Biochem. Soc. Symp. 50:43–58
Maldonado, P.E., Rose, B., Loewenstein, W.R. 1988. Growth factors modulate junctional cell-to-cell communication. J. Membrane Biol. 106:203–210
Meda, P., Bosco, D., Giordano, E., Chanson, M. 1991. Junctional coupling modulation by secretagogues in two-cell pancreatic systems. In: Biophysics of Gap Junction Channels. C. Peracchia, editor, pp. 191–208. CRC Press, Boca Raton
Mehta, P.P., Yamamoto, Y., Rose, B. 1992. Transcription of the gene for the gap junction protein connexin43 and expression of functional cell-to-cell channels are regulated by cAMP. Molec. Biol. Cell 3:839–850
Moreno, A.P., Sáez, J.C., Fishman, G.I., Spray, D.C. 1994. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ. Res. 74:1050–1057
Musil, L.S., Goodenough, D.A. 1993. Phosphorylation, intracellular transport, and assembly into gap junctions of connexin 43. In: Progress in Cell Research, Vol. 3. Gap Junctions. J.E. Hall, G.A. Zampighi and R.M. Davis, editors, pp. 255–262. Elsevier Science Publishers, Amsterdam
Nnamani, C., Godwin, A., Ducsay, C.A., Longo, L.D., Fletcher, W.H. 1994. Regulation of cell-cell communication mediated by connexin 43 in rabbit myometrial cells. Biol. Reprod. 50:377–389
Pelletier, D.B., Boynton, A.L. 1994. Disassociation of PDGF receptor tyrosine kinase activity from PDGF-mediated inhibition of gap junction communication. J. Cell. Physiol. 158:427–434
Petrocelli, T., Lye, S.J. 1993. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology 133:284–290
Pluciennik, F., Joffre, M., Délèze, J. 1994. Follicle-stimulating hormone increases gap junction communication in Sertoli cells from immature rat testis in primary culture. J. Membrane Biol. 139:81–96
Pressler, M.L., Hathaway, D.R. 1987. Phosphorylation of purified dog heart gap junctions by cAMP dependent protein kinase. Circulation 76:18
Purnam, K.L., Laird, D.W., Revel, J.-P. 1993. Trapping an intermediate form of connexin 43 in the Golgi. Exp. Cell Res. 206:85–92
Rogers, M., Berestecky, J., Hossain, M., Gwo, H., Kadle, R., Nicholson, B., Bertram, J.S. 1990. Retanoid-enhanced gap junctional communication is achieved by increasing levels of connexin 43 mRNA and protein. Molec. Carcinog. 3:335–343
Saez, J.C., Berthoud, V.M., Moreno, A.P., Spray, D.C. 1993. Gap Junctions. Multiplicity of controls in differentiated and undifferentiated cells and possible functional implications. In: Advances in Second Messenger and Phosphoprotein Research. Volume 27. S. Shenolikar and A.C. Narin, editors, pp. 163–198. Raven Press, New York
Saez, J.C., Nairn, A.C., Czernick, A., Spray, D.C., Hertzberg, E.L., Greengard, P., Bennett, M.V.L. 1990. Phosphorylation of connexin 32, the hepatocyte gap junction protein, by cAMP-dependent protein kinase, protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Eur. J. Biochem. 192:263–273
Sakai, N., Blennerhassett, M.G., Garfield, R.E. 1992. Intracellular cyclic AMP concentration modulates gap junction permeability in parturient rat myometrium. Can. J. Physiol. Pharmacol. 70:358–364
SAS Institute, 1985. SAS/STAT Guide for Personal Computers. SAS Institute, Cary, NC
Schelling, M.E., Meininger, C.J., Hawker, J.R., Granger, H.J. 1988. Venular endothelial cells from bovine heart. Am. J. Physiol. 254:H1211-H1217
Schiller, P.C., Mehta, P.P., Roos, B.A., Howard, G.A. 1992. Hormonal regulation of intercellular communication: parathyroid hormone increases connexin 43 gene expression and gap-junctional communication in osteoblastic cells. Molec. Endocrinol. 6:1433–1440
Somogyi, R., Kolb, H.-A. 1988. Cell-to-cell channel conductance during loss of gap junctional coupling in pairs of pancreatic acinar and Chinese hamster ovary cells. Pfluegers Arch. 412:54–65
Stagg, R.B., Fletcher, W.H. 1990. The hormone-induced regulation of contact-dependent cell-cell communication by phosphorylation. Endocrine Rev. 11:302–325
Stein, L.S., Boonstra, J., Burghardt, R.C. 1992. Reduced cell-cell communication between mitotic and nonmitotic coupled cells. Exp. Cell Res. 198:1–7
Stein, L.S., Stein, D.W.J., Echols, J.E., Burghardt, R.C. 1993. Concomitant alteration of desmosomes, adhesiveness, and diffusion through gap junction channels in a rat ovarian transformation model system. Exp. Cell Res. 207:19–32
Stein, L.S., Stoica, G., Tilley, R., Burghardt, R.C. 1991. Rat ovarian granulosa cell culture: a model system for the study of cell-cell communication during multistep transformation. Cancer Res. 51:696–706
Swenson, K.I., Piwinca-Worms, H., McNamee, H., Paul, D.L. 1990. Tyrosine phosphorylation of the gap junction protein connexin43 is required for the pp60v-src-induced inhibition of communication. Cell Regulation 1:989–1002
Wade, M.A., Trosko, J.E., Schindler, M. 1986. A fluorescence photo-bleaching assay of gap junction-mediated communication between human cells. Science 232:525–528
Willecke, K., Hennemann, H., Dahl, E., Jungbluth, S., Heynkes, R. 1991. The diversity of connexin genes encoding gap junctional proteins. Eur. J. Cell Biol. 56:1–7
Winterhager, E., Stutenkemper, R., Traub, O., Beyer, E., Willecke, K. 1991. Expression of different connexin genes in rat uterus during decidualization and at term. Eur. J. Cell Biol. 55:133–142
Yu, W., Dahl, G., Werner, R. 1994. The connexin43 gene is responsive to oestrogen. Proc. R. Soc. Land. B. 255:125–132
Author information
Authors and Affiliations
Additional information
We acknowledge the technical assistance of Richard Lewis and Meghan Abella. We thank Dr. Hugh Dookwah for contributions to the myometrial cell isolation protocol and Drs. Stephen H. Safe, Timothy D. Phillips, and Evelyn Tiffany-Castiglioni for helpful discussions. This work was funded by NIH (HD-26182, P42-ES04917, ES05871-01A1), the March of Dimes Birth Defects Foundation Basic Research grant #1-0796, and USDA 92-37203-7952.
Rights and permissions
About this article
Cite this article
Burghardt, R.C., Barhoumi, R., Sewall, T.C. et al. Cyclic AMP induces rapid increases in gap junction permeability and changes in the cellular distribution of connexin43. J. Membarin Biol. 148, 243–253 (1995). https://doi.org/10.1007/BF00235042
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00235042