Abstract
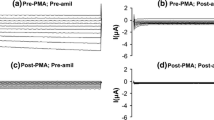
Previous studies indicate that inhibition of protein N-glycosylation reduces Na+-pump activity. Since this effect is preceded by an inhibition of the entry of sodium into the cell, it is unclear whether the reduced Na+-pump is produced by the inactivation of protein glycosylation per se or by the lower intracellular sodium concentration. We compared the effects of tunicamycin, which inhibits protein glycosylation, and amiloride, which inhibits the entry of sodium into the cell, on the expression of the Na+-pump activity in A6 cells. The short-circuit current across A6 epithelia, which corresponds to sodium ions transported through the Na+ channel and the Na+-pump, was almost totally inhibited after 24-hr treatment with 1 μg/ml tunicamycin. The maximal Na+-pump activity, measured after permeabilizing the apical cell membrane with amphotericin B, was only 30% inhibited. This inhibition increased to 80% after 72-hr treatment with tunicamycin. Thus, tunicamycin inhibits the activities of both the apical Na+ channel and the basolateral Na+-pump. However, the reduced number of Na+-pump molecules, as well as the inhibition of the Na+-pump activity, were not observed when the Na+ channel was inhibited for 72-hr with amiloride. Thus, the reduced Na+-pump expression produced by inactivation of protein glycosylation is not secondary to reduced entry of sodium into the cell.
Similar content being viewed by others
References
Alboim, S.V., Bak, A., Sampson, S.R. 1992. Tunicamycin reduces Na+, K+-ATPase-pump expression in cultured skeletal muscle. J. Cell. Physiol. 150:640–646
Brodie, C., Sampson, S.R. 1989. Regulation of the sodium-potassium pump in cultured rat skeletal myotubes by intracellular sodium ions. J. Cell. Physiol. 140:131–137
Cass, A., Finkelstein, A., Krespi, V. 1970. The ion permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J. Gen. Physiol. 56:100–124
Churchill, L., Hokin, L.E. 1976. The susceptibility of the glycoprotein from the purified (Na+, K+)-activated adenosine triphosphatase to tryptic and chymotryptic degradation with and without Na+ and K+. Biochem. Biophys. Acta 434:258–264
Forbush III, B. 1983. Assay of Na,K-ATPase in plasma membrane preparations: increasing permeability of membrane vesicles using sodium dodecyl sulfate buffered with bovine serum albumin. Anal. Biochem. 128:159–163
Frizzell, R.A., Schultz, S.G. 1978. Effect of aldosterone on ion transport by rabbit colon in vitro. J. Membrane Biol. 39:1–26
Garty, H., Benos, D.J. 1988. Characteristics and regulatory mechanisms of the amiloride-blockage Na channel. Physiol. Rev. 68:309–373
Geering, K. 1990. Subunit assembly and functional maturation of Na,K-ATPase. J. Membrane Biol. 115:109–121
Geering, K., Meyer, D.I., Paccolat, M.P., Kraehenbuhl, J.P., Rossier, B.C. 1985. Membrane insertion of α-and β-subunits of Na,K-ATPase. J. Biol. Chem. 260:5154–5160
Graf, J., Giebisch, G. 1979. Intracellular sodium activity and sodium transport in Necturus gallbladder epithelium. J. Membrane Biol. 47:327–355
Handler, J.S. 1983. Use of cultured epithelia to study transport and its regulation. J. Exp. Biol. 106:55–69
Jorgensen, P.L., Anderson, J.P. 1988. Structural basis for E1–E2 conformational transitions in Na,K-pump and Ca-pump proteins. J. Membrane Biol. 103:95–120
Katz, A.I. 1982. Renal Na,K-ATPase: its role in tubular sodium and potassium transport. Am. J. Physiol. 242:F207-F219
Laemmli, B.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee, J.A., Fortes, P.A.G. 1985. Labeling of the glycoprotein subunit of (Na,K)ATPase with fluorescent probes. Biochemistry 24:322–330
Olden, K., Pratt, R.M., Jaworski, C., Yamada, K.M. 1979. Evidence for role of glycoprotein carbohydrates in membrane transport: specific inhibition by tunicamycin. Proc. Natl. Acad. Sci. USA 76:791–795
Pedemonte, C.H., Kaplan, J.H. 1986. Carbodiimide inactivation of Na,K-ATPase: A consequence of internal cross-linking and not carboxyl group modification. J. Biol. Chem. 261:16660–16665
Pedemonte, C.H., Kaplan, J.H. 1990. Chemical modification as an approach to elucidation of sodium pump structure-function relations. Am. J. Physiol. 258:C1-C23
Pedemonte, C.H., Kaplan, J.H. 1992. A monosaccharide is bound to the Na-pump α-subunit. Biochemistry 31:10465–10470
Pedemonte, C.H., Sachs, G., Kaplan, J.H. 1990. An intrinsic membrane glycoprotein with cytosolically oriented N-linked sugars. Proc. Natl. Acad. Sci. USA 87:9789–9793
Perkins, F.M., Handler, J.S. 1981. Transport properties of toad kidney epithelia in culture. Am. J. Physiol. 241:C154-C159
Pressley, A.T. 1988. Ion concentration-dependent regulation of Na,K-pump abundance. J. Membrane Biol. 105:187–195
Takeda, K., Noguchi, S., Atsuko, S., Kawamura, M. 1988. Functional activity of oligosaccharide-deficient (NaK)ATPase expressed in Xenopus oocytes. FEBS Lett. 238:201–204
Tamkun, M.M., Fambrough, D.M. 1986. The Na,K-ATPase of chick sensory neurons. J. Biol. Chem. 261:1009–1019
Verrey, F., Kairouz, P., Schaerer, E., Fuentes, P., Geering, K., Rossier, B.C., Kraehenbuhl, J. 1989. Primary sequence of Xenopus laevis Na,K-ATPase and its localization in A6 kidney cells. Am. J. Physiol. 256:F1034-F1043
Wolitzky, B.A., Fambrough, D.M. 1986. Regulation of the Na,K-ATPase in cultured chick skeletal muscle. Modulation of expression by the demand for ion transport. J. Biol. Chem. 261:9990–9999
Zamofing, D., Rossier, B.C., Geering, K. 1988. Role of the Na,K-ATPase β-subunit in the cellular accumulation and maturation of the enzyme as assessed by glycosylation inhibitors. J. Membrane Biol. 104:69–79
Zamofing, D., Rossier, B.C., Geering, K. 1989. Inhibition of N-glycosylation affects transepithelial Na+ but not Na+, K+-ATPase transport. Am. J. Physiol. 256:C958-C966
Author information
Authors and Affiliations
Additional information
The author wants to thank Angel Chinelli (NYU) for his help with the SCC measurements; Thomas Kleyman (University of Pennsylvania) in whose laboratory some of the SCC measurements were done; Michael Kaplan (Yale University) for the monoclonal antibody; and Douglas Eikenburg, Mustafa Lokhandwala and Charlotte Tate for their critique of the manuscript. This work was supported by a grant from the National Science Foundation.
Rights and permissions
About this article
Cite this article
Pedemonte, C.H. Inhibition of Na+-pump expression by impairment of protein glycosylation is independent of the reduced sodium entry into the cell. J. Membarin Biol. 147, 223–231 (1995). https://doi.org/10.1007/BF00234520
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00234520