Summary
The cellular mechanisms responsible for rectal acidification in the desert locust, Schistocerca gregaria, were investigated in isolated recta mounted as flat sheets in modified Ussing chambers. Previous studies conducted in the nominal absence of exogenous CO2 and HCO −3 suggested that the acidification was due to a proton-secretory rather than bicarbonate-reabsorptive mechanism (Thomson, R.B., Speight, J.D., Phillips, J.E. 1988. J. Insect Physiol. 34:829–837). This conclusion was confirmed in the present study by demonstrating that metabolic CO2 could not contribute sufficient HCO −3 to the lumen to account for the rates of rectal acidification observed under the nominally CO2/ HCO −3 -free conditions used in these investigations.
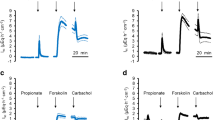
Rates of luminal acidification (J H +) were completely unaffected by changes in contraluminal pH, but could be progressively reduced (and eventually abolished) by imposition of either transepithelial pH gradients (lumen acid) or transepithelial electrical gradients (lumen positive). Under short-circuit current conditions, the bulk of J H + was not dependent on Na+, K+, Cl−,Mg2+, or Ca2+ and was due to a primary electrogenic proton translocating mechanism located on the apical membrane. A small component (10–16%) of J H + measured under these conditions could be attributed to an apical amiloride-inhibitable Na+/H+ exchange mechanism.
Similar content being viewed by others
References
Al-Awqati, Q., Mueller, A., Steinmetz, P.R. 1977. Transport of H against electrochemical gradients in turtle urinary bladder. Am. J. Physiol. 233:F502-F508
Aronson, P.S. 1983. Mechanisms of active H secretion in the proximal tubule. Am. J. Physiol. 245:F647-F659
Arruda, J.A.L., Sabatini, S., Westenfelder, C. 1981. Vanadate inhibits urinary acidification by the turtle bladder. Kidney Int. 20:772–779
Balshin, M., Phillips, J.K. 1971. Active absorption of amino acids in the rectum of the desert locust (Schistocerca gregaria). Nature 233:53–55
Beauwens, R., Crabbe, J., Rentmeesters, M. 1981. Effects of vanadate on the functional properties of the isolated toad bladder. J. Physiol. 310:293–305
Black, K.T., Meredith, J., Thomson, B., Phillips, J., Dietz, T. 1987. Mechanisms and properties of sodium transport in locust rectum. Can. J. Zool. 65:3084–3092
Brodsky, W.A., Schilb, T.P. 1974. The means of distinguishing between hydrogen secretion and bicarbonate reabsorption: Theory and applications to the reptilian bladder and mammalian kidney. In: Current Topics in Membranes and Transport. F. Bonner and A. Kleinzeller, editors. Vol. 4, pp. 162–224. Academic, New York
Burckhardt, B.C., Fromter, E. 1987. Evidence for OH /H per meation across the peritubular cell membrane of rat renal proximal tubule in HCO3-free solutions. Pfluegers Arch. 409:132–137
Chamberlin, M.E. 1981. Metabolic Studies on the Locust Rectum. Ph.D. Thesis. University of British Columbia, Vancouver
Chamberlin, M.E., Phillips, J.E. 1982. Metabolic support of chloride-dependent short-circuit current across locust rectum. J. Exp. Biol. 99:349–361
Curci, S., Debellis, L., Fromter, E. 1987. Evidence for rheogenic sodium bicarbonate cotransport in the basolateral membrane of oxyntic cells of frog gastric fundus. Pfluegers Arch. 408:497–504
Dilley, R.A., Giaquinta, R.T. 1975. H ion transport and energy transduction in chloroplasts. In: Current Topics in Membranes and Transport. F. Browner and A. Kleinzeller, editors. Vol. 7, pp. 49–107. Academic, New York
DuBose, T.D. 1983. Application of the disequilibrium pH method to investigate the mechanism of urinary acidification. Am. J. Physiol. 2245:F535-F544
Fischer, J.L., Husted, R.F., Steinmetz, P.R. 1983. Chloride dependence of the HCO3 exit step in urinary acidification by the turtle bladder. Am. J. Physiol. 245:F564-F568
Forte, J.G., Machin, T.E., 1987. Ion transport by gastric mucosa. In: Membrane Physiology. T.E. Andreoli, J.F. Hoffman, D.D. Fanestil, and S.G. Schultz, editors, pp. 151–174. Plenum, New York
Giebisch, G., Aronson, P.S. 1987. The proximal nephron. In:Membrane Transport Processes in Organized Systems. T.E. Andreoli, J.F. Hoffman, D.D. Fanestil, and S.G. Schultz, editors. pp. 285–316. Plenum, New York
Gimenez-Gallego, G., Benavides, J., Garcia, M.I., Valdivieso, F. 1980. Occurrence of a reduced nicotinamidc adcninc dinucleotidc oxidase activity linked to a cytochrome system in renal brush border membranes. Biochemistry 19:4834–4839
Gluck, S., Cannon, C., Al-Awqati, Q. 1982. Flxocytosis regulates urinary acidification in turtle bladder by rapid insertion of H pumps into the luminal membrane. Proc. Natl. Acad. Sci.USA 79:4327–4331
Grassl, S.M., Aronson, P.S. 1986. Na+/HCO3 co-transport in basolateral membrane vesicles isolated from rabbit renal cortex. J. Biol. Chem. 261:8778–8783
Hanrahan, J.W. 1982. Cellular Mechanisms and Regulation of KCI Transport across an Insect Epithelium. Ph.D. Thesis. University of British Columbia, Vancouver
Hanrahan, J.W., Meredith, J., Phillips, J.E., Brandys, D. 1984. Methods for the study of transport and control in insect hindgut. In: Measurement of Ion Transport and Metabolic Rate in Insects. T.J. Bradley and T.A. Miller, editors, pp. 19–68. Springer-Verlag, New York
Hanrahan, J.W., Phillips, J.E. 1983. Mechanism and control of salt absorption in locust rectum. Am. J. Physiol. 224:R131-R142
Hanrahan, J.W., Phillips, J.E. 1984a. KCI transport across an insect epithelium: I. Tracer fluxes and the effects of ion substitutions. J. Membrane Biol. 80:15–26.
Hanrahan, J.W., Phillips, J.E. 1984b. KCI transport across an insect epithelium: II. Electrical potentials and electrophysiology. J. Membrane Biol. 80:27–47
Harold, F.M., Altendorf, K. 1974. Cation transport in bacteria:K, Na, and H. In: Current Topics in Membranes and Transport. F. Browner and A. Kleinzeller, editors. Vol. 5, pp.1–49. Academic, New York
Jentsch, T.J., Keller, S.K., Koch, M., Wiederholt, M. 1984. Evidence for coupled transport of bicarbonate and sodium in cultured bovine corncal endothelial cells. J. Membrane Biol. 81:189–204
Kinne-Saffran, F., Kinne, R. 1986. Proton pump activity and Mg-ATPasc activity in rat kidney cortex brush border membranes:Effect of ‘proton ATPase’ inhibitors. Pfluegers Arch. 407:S180-S185
Kinsella, J.L., Aronson, P.S. 1980. Properties of the Na/H exchanger in renal microvillus membrane vesicles. Am. J.Physiol. 238:F461-F469
Knauf, P.A. 1987. Anion transport in erythrocytes. In: Membrane Physiology. T.E. Andreoli, J.F. Hoffman, D.D. Fanestil, and S.G. Schultz, editors, pp. 191–220. Plenum, New York
Kuwabara, M., Ishibashi, K., Krapf, R., Rector, F.C., Berry, C.A. 1989. Effect of lumen pH on cell pH and cell potential in rabbit proximal tubules. Am. J. Physiol. 256:F1075-F1083
Lechleitner, R.A. 1988. Properties of ion and fluid transport and control in hindgut of the desert locust (Schistocerca gregaria). Ph.D. Thesis. University of British Columbia, Vancouver
Pedersen, P.L., Carafoli, E. 1987. Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem. Sci. 12:146–150
Phillips, J.E. 1961. Studies on the rectal absorption of water and salts in the locust, Schistocerca gregaria, and the blowfly, Calliphora erythrocephala. Ph.D. Thesis. University of Cambridge, England
Phillips, J.E. 1981. Comparative physiology of insect renal function. Am. J. Physiol. 241:R241-R257
Phillips, J.E., Hanrahan, J.W., Chamberlin, M.E., Thomson, R.B. 1986. Mechanisms and control of reabsorption in insect hindgut. In: Advances in Insect Physiology, P.D. Evans and V.B. Wigglesworth, editors. Vol. 19, pp. 329–422. Academic, London
Preisig, P.A., Alpern, R.J. 1989. Basolateral membrane H-OH-HCO3 transport in the proximal tubule. Am. J. Physiol. 256:F751-E765
Ramsay, J.A., Brown, R.H.J., Croghan, P.C. 1955. Electrometric titration of chloride in small volumes. J. Exp. Biol. 32:822–829
Rector, F.C., Carter, N.M., Seldin, D.W. 1965. The mechanisms of bicarbonate reabsorption in the proximal and distal tubules of the kidney. J. Clin. Invest. 44:278–290
Rehm, W.S. 1972. Proton transport. In: Metabolic Pathways. L.E. Hokin, editor. Vol. 6, pp. 187–241. Academic, New York
Reuss, L., Constantin, J.L. 1984. Cl/HCO3 exchange at the apical membrane of Necturus gallbladder. J. Gen. Physiol. 83:801–818
Schilb, T.P. 1978. Bicarbonate ion transport: A mechanism for the acidification of urine in the turtle. Science 220:208–209
Schilb, T.P., Durham, J.H., Brodsky, W.A. 1988. In vivo environmental temperature and the in vitro pattern of luminal acidification in turtle bladders. Evidence for HCO3 ion reabsorption. J. Gen. Physiol. 92:613–642
Schwartz, G.J., Weinstein, A.M., Steele, R.E., Slephenson, J.L., Burg, M.B. 1981. Carbon dioxide permeability of rabbit proximal convoluted tubules. Am. J. Physiol. 240:F231:F244
Schwartz, J.H., Finn, J.T., Vaughan, G., Steinmetz, P.R. 1974. Distribution of metabolic CO2 and the transported ion species in acidification by turtle bladder. Am. J. Physiol. 226:283–289
Schwartz, J.H., Steinmetz, P.R. CO2 requirements for H+ secretion by the isolated turtle bladder. Am. J. Physiol. 220:2051–2057
Schweikl, H., Klein, U., Schindlbeck, M., Wieczorck, H. 1989. A vacuolar-type ATPase, partially purified from potassium transporting plasma membranes of tobacco hornworm midgut. J. Biol. Chem. 264:11136–11142
Seifter, J.L. Aronson, P.S. 1986. Properties and physiologic roles of the plasma membrane sodium-hydrogen exchanger. J. Clin.Invest. 78:859–864
Siggaard-Andersen, O. 1976. The Acid-Base Status of the Blood. (4th ed.) pp. 1–83. Williams and Wilkins, Baltimore
Speight, J. 1967. Acidification of rectal fluid in the locust, Schistocerca gregaria. M.Sc. Thesis. University of British Columbia, Vancouver
Stagg, A.P., Harrison, J.F., Phillips, J.E. 1991. Acid-base parameters in Malpighian tubule secretion and response to acidosis. J. Exp. Biol. (in press)
Steinmetz, P.R. 1969. Acid-base relations in epithelium of turtle bladder: Site of active step in acidification and role of metabolic CO2. J. Clin. Invest. 48:1258–1265
Steinmetz, P.R. 1974. Cellular mechanisms of urinary acidification. Physiol. Rev. 54:890–956
Steinmetz, P.R., Andersen, O.S. 1982. Electrogenic proton transport in epithelial membranes. J. Membrane Biol. 65:155–174
Steinmetz, P.R., Husted, R.F., Mueller, A., Beauwens, R. 1981. Coupling between H transport and anaerobic glycolysis in turtle urinary bladder: Effects of inhibitors of H+ ATPase. J.Membrane Biol. 59:27–34
Strange, K., Phillips, J.E. 1984. Mechanisms of CO2 transport in rectal salt gland of Aedes. I. Ionic requirements of CO2 secretion. Am. J. Physiol. 246:R727-R734
Strange, K., Phillips, J.E. 1985. Cellular mechanisms of HCO3and Cl transport in insect salt gland. J. Membrane Biol. 83:25–37
Thomson, R.B. 1990. Cellular mechanisms of acid/base transport in an insect excretory epithelium. Ph.D. Thesis. University of British Columbia, Vancouver
Thomson, R.B., Audsley, N., Phillips, J.E. 1991. Acid-base transport and control in locust hindgut: Artifacts caused by short-circuit current. J. Exp. Biol. 155:455–467
Thompson, R.B., Phillips, J.K. 1985. Characterization of acid/base transport in an insect epithelium. Fed. Proc. 44:1361
Thomson, R.B., Speight, J.D., Phillips, J.E. 1988a. Rectal acid secretion in the desert locust, Schistocerca gregaria. J. Insect Physiol. 34:829–837
Thomson, R.B., Thomson, J.M., Phillips, J.E. 1988b. NH +4 transport in an acid secreting insect epithelium. Am. J. Physiol. 254:R348-R356
Turrini, F., Sabolic, I., Zimolo, Z., Moewes, B., Burckhardt, G. 1989. Relation of ATPases in rat renal brush-border membranes to ATP-driven H+ secretion. J. Membrane Biol. 107:1–12
Wikstrom, M. 1982. Proton translocation by cytochrome oxidase. Curr. Top. Membr. Transp. 16:303–321
Williams, D., Phillips, J.E., Prince, W.T., Meredith, J. 1977. The source of short-circuit current across locust rectum. J. Exp.Biol. 77:107–122
Zeidel, M.L., Silva, P., Seifter, J.L. 1986a. Intracellular pH regulation and proton transport by rabbit renal medullary collecting duct cells: Role of plasma membrane proton adenosine triphosphatase. J. Clin. Invest. 77:113–120
Zeidel, M.L., Silva, P., Seifter, J.L. 1986b. Intracellular pH regulation in rabbit renal medullary collecting duct cells: Role of chloride-bicarbonate exchange. J. Clin. Invest. 77:1682–1688
Author information
Authors and Affiliations
Additional information
This work was supported by operating grants to J.E.P. and postgraduate scholarships to R.B.T. from Natural Sciences & Engineering Research Council, Canada.
Rights and permissions
About this article
Cite this article
Thomson, R.B., Phillips, J.E. Electrogenic proton secretion in the Hindgut of the desert locust, Schistocerca gregaria . J. Membarin Biol. 125, 133–154 (1992). https://doi.org/10.1007/BF00233353
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00233353