Abstract
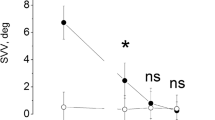
Rapid, passive, unpredictable, low-amplitude (10–20°), high-acceleration (3000–000°/s2) head rota tions were used to study the vertical vestibulo-ocular reflex in the pitch plane (pitch-vVOR) after unilateral vestibular deafferentation. The results from 23 human subjects who had undergone therapeutic unilateral vestibular deafferentation were compared with those from 19 normals. All subjects were tested while seated in the upright position. Group means and two-tailed 95% confidence intervals are reported for the pitch-vVOR gains in normal and unilateral vestibular deafferented subjects. In normal subjects, at a head velocity of 125°/s the pitch-vVOR gains were: upward 0.89±0.06, down ward 0.91±0.04. At a head velocity of 200°/s, the pitchvVOR gains were: upward 0.92±0.06, downward 0.96±0.04. There was no significant up-down asymme try. In the 15 unilateral vestibular deafferented subjects who were studied more than 1 year after unilateral vestibular deafferentation, the pitch-vVOR was signifi cantly impaired. At a head velocity of 125°/s the pitchvVOR gains were: upward 0.67±0.11, downward 0.63 ± 0.07. At a head velocity of 200°/s, the pitch-vVOR gains were: upward 0.67±0.07, downward 0.58±0.06. There was no significant up-down asymmetry. The pitch-vVOR gain in unilateral vestibular deafferented subjects was significantly lower (P<0.05) than the pitch-vVOR gain in normal subjects at the same head velocities. These results show that total, permanent uni lateral loss of vestibular function produces a permanent symmetrical 30% (approximately) decrease in pitchv-VOR gain. This pitch-vVOR deficit is still present more than 1 year after deafferentation despite retinal slip velocities greater than 30°/s in response to head accelerations in the physiological range, indicating that compensation of pitch-vVOR function following unilat eral vestibular deafferention remains incomplete.
Similar content being viewed by others
References
Allum JHJ, Yamane M, Pfaltz CR (1988) Long-term modifica tions of vertical and horizontal vestibulo-ocular reflex dynamics in man. Acta Otolaryngol 105:328–337
Bahill AT, Kallman JS, Lieberman JE (1982) Frequency limita tions of the two-point central difference differentiation algorithm. Biol Cybern 45:1–4
Baloh RW, Demer J (1991) Gravity and the vertical vestibul-ocular reflex. Exp Brain Res 83:427–433
Baloh RW, Rickman L, Yee RD, Honrubia V (1983) The dynam ics of vertical eye movements in normal human subjects. Aviat Space Environ Med 54:32–38
Baloh RW, Honrubia V, Yee RD, Jacobson K (1986) Vertical visu al interaction in normal human subjects. Exp Brain Res 64:400–406
Barr CC, Schultheis LW, Robinson DA (1976) Voluntary, non-visual control of the human vestibulo-ocular reflex. Acta Otolar yngol 81:365–375
Becker RA, Chambers JM, Wilks AR (1988) The new S language. A programming environment for data analysis and graphics. AT&T Bell Laboratories. (Computer science series) Wadsworth & Brooks/Cole, Pacific Grove, Calif.
Black FO, Shupert CL, Peterka RJ, Nashner LM (1989) Effects of unilateral loss of vestibular function on the vestibulo-ocular reflex and postural control. Ann Otol Rhinol Laryngol 98:884–889
Bronstein AM, Hood JD (1986) The cervico-ocular reflex in normal subjects and patients with absent vestibular function. Brain Res 373:399–408
Carl JR, Gellman RS (1987) Human smooth pursuit: stimulus-dependent responses. J Neurophysiol 57:1446–1463
Chae S, Igarashi M, Kim BW (1990) Compensation of vertical vestibulo-ocular functions in squirrel monkeys after unilateral labyrinthectomy. Am J Otolaryngol 11:170–173
Cleveland WS (1979) Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 74:829–836
Collewijn H (1989a) The vestibulo-ocular reflex: is it an independent subsystem? Rev Neurol (Paris) 145:502–512
Collewijn H (1989b) The vestibulo-ocular reflex: an outdated con cept? Prog Brain Res 80:197–209
Collewijn H, Martins AJ, Steinman RM (1983) Compensatory eye movements during active and passive head movements: fast adaptation to changes in visual magnification. J Physiol (Lond) 340:259–286
Correia MJ, Perachio AA, Eden AR (1984) The effects of gravity on the vertical vestibulo-ocular response of the monkey. Physiologist 27: S87-S88
Curthoys IS, Dai MJ, Halmagyi GM (1991) Human ocular torsion position before and after unilateral vestibular neurectomy. Exp Brain Res 85:218–225
Dai MJ, Curthoys IS, Halmagyi GM (1989) Linear acceleration perception in the roll plane before and after unilateral vestibular neurectomy. Exp Brain Res 77:315–328
Darlot C, Löpez-Barneo J, Tracey D (1981) Asymmetries of verti cal vestibular nystagmus in the cat. Exp Brain Res 41:420–426
Demer JL (1992) Mechanism of human vertical visual-vestibular interaction. J Neurophysiol 68:2128–2146
Demer JL, Amjadi F (1993) Dynamic visual acuity of normal subjects during vertical optotype and head motion. Invest Ophthalmol Vis Sci 34:1894–1906
Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE (1988) Frequency and velocity of rotational head perturba tions during locomotion. Exp Brain Res 70:470–476
Grossman GE, Leigh RJ, Bruce EN, Huebner WP, Lanska DJ (1989) Performance of the human vestibuloocular reflex during locomotion. J Neurophysiol 62:264–271
Halmagyi GM, Curthoys IS, Cremer PD, Henderson CJ, Todd MJ, Staples MJ, D'Cruz DM (1990) The human horizontal vestibulo-ocular reflex in response to high-acceleration stimu lation before and after unilateral vestibular neurectomy. Exp Brain Res 81:479–490
Halmagyi GM, Aw ST, Cremer PD, Todd MJ, Curthoys IS (1992) The human vertical vestibuloocular reflex in response to highacceleration stimulation after unilateral vestibular neurectomy. Ann NY Acad Sci 656:732–738
Istl-Lenz Y, Hyden D, Schwarz DWF (1985) Response of the human vestibuloocular reflex following long-term 2 × magnified visual input. Exp Brain Res 57:448–455
Leigh RJ, Brandt T (1993) A reevaluation of the vestibulo-ocular reflex: new ideas of its purpose, properties, neural substrate and disorders. Neurology 43:1288–1295
Lisberger SG (1988) The neural basis for motor learning in the vestibulo-ocular reflex in monkeys. Trends Neurosci 11:147–152
Matsuo V, Cohen B (1984) Vertical optokinetic nystagmus vestibular nystagmus in the monkey: up-down asymmetry and effects of gravity. Exp Brain Res 53:197–216
Maioli C, Precht W (1985) On the role of vestibulo-ocular reflex plasticity in recovery after unilateral peripheral vestibular lesion. Exp Brain Res 59:267–272
Melvill-Jones G, Gonshor A (1982) Oculomotor response to rapid head oscillation (05–50 Hz) after prolonged adaptation to vision-reversal “simple” and “complex” effects. Exp Brain Res 45:45–58
Melvill-Jones G, Segal B, Katsarkas A, Bloomberg J (1988) Experiments on vestibular adaptation and its clinical significance. In: Barber HO, Sharpe JA (eds) Vestibular disorders. Year Book Medical, Chicago
Robinson DA (1963) A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng BME-10:137–145
Segal BN, Katsarkas A (1988) Goal-directed vestibulo-ocular function in man: gaze stabilization by slow-phase and saccadic eye movements. Exp Brain Res 70:26–32
Tomko DL, Wall C, Robinson FR, Staab JP (1987) Gain phase of cat vertical eye movements generated by sinusoidal pitch rotations with and without head tilt. Aviat Space Environ Med 58: A186–188
Tomko DL, Wall C, Robinson FR, Staab JP (1988) Influence of gravity on cat vertical vestibulo-ocular reflex. Exp Brain Res 69:307–314
Tomlinson RD, Saunders GE, Schwarz DWF (1980) Analysis of human vestibulo-ocular reflex during active head movements. Acta Otolaryngol 90:184–190
Westheimer G, McKee SP (1975) Visual acuity in the presence of retinal image motion. J Opt Soc Am [A] 65:847–850
Winer BJ, Brown DR, Michels KM (1991) Statistical principles in experimental design, 3rd edn. McGraw-Hill, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aw, S.T., Halmagyi, G.M., Curthoys, I.S. et al. Unilateral vestibular deafferentation causes permanent impairment of the human vertical vestibulo-ocular reflex in the pitch plane. Exp Brain Res 102, 121–130 (1994). https://doi.org/10.1007/BF00232444
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00232444