Summary
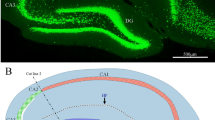
The relationship between neuronal calcium binding protein content (calbindin D28K: CaBP and parvalbumin : PV) and vulnerability to ischemia was studied in different regions of the rat brain using the four vessel occlusion model of complete forebrain ischemia. The areas studied, i.e. the hippocampal formation, neocortex, neostriatum and reticular thalamic nucleus (RTN), show a characteristic pattern of CaBP and PV distribution, and are involved in ischemic damage to different degrees. In the hippocampal formation CaBP is present in dentate granule cells and in a subpopulation of the CA1 pyramidal cells, the latter being the most and the former the least vulnerable to ischemia. Non-pyramidal cells containing CaBP in these regions survive ischemia, whereas PV-containing non-pyramidal cells in the CA1 region are occasionally lost. Hilar somatostatin-containing cells and CA3 pyramidal cells contain neither PV nor CaBP. Nevertheless, the latter are resistant to ischemia and the former is the first population of cells that undergoes degeneration. Supragranular pyramidal neurons containing CaBP are the most vulnerable cell group in the sensory neocortex. In the RTN the degenerating neurons contain both PV and CaBP. In the neostriatum, ischemic damage involves both CaBP-positive and negative medium spiny neurons, although the degeneration always starts in the dorsolateral neostriatum containing relatively few CaBP-positive cells. The giant cholinergic interneurons of the striatum contain neither CaBP nor PV, and they are the most resistant cell type in this area. These examples suggest the lack of a consistent and systematic relationship between neuronal CaBP or PV content and ischemic vulnerability. It appears that some populations of cells containing CaBP or PV are more predisposed to ischemic cell death than neurons lacking these proteins. These neurons may express high levels of calcium binding proteins because their normal activity may involve a high rate of calcium uptake and/or intraneuronal release.
Similar content being viewed by others
References
Andine P, Jacobson I, Hagberg H (1988) Calcium uptake evoked by electrical stimulation is enhanced postischemically and precedes delayed neuronal death in CA1 of rat hippocampus: involvement of N-methyl-D-aspartate receptors. J Cereb Blood Flow Metab 8:799–807
Baimbridge KG, Kao J (1988) Calbindin D-28K protects against glutamate induced neurotoxicity in rat CA1 pyramidal neuron cultures. Soc Neurosci Abstr 14:1264
Baimbridge KG, Miller JJ (1982) Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res 245:223–229
Baimbridge KG, Miller JJ, Parkes CO (1982) Calcium-binding protein distribution in the rat brain. Brain Res 239:519–525
Baudry M, Bundman M, Smith E, Lynch G (1981) Micromolar calcium stimulates proteolysis and glutamate binding in rat brain synaptic membranes. Science 212:937–938
Blaxter TJ, Carlen PL, Davies MF, Kujtan PW (1986) γ-Aminobutyric acid hyperpolarizes rat hippocampal pyramidal cells through a calcium-dependent potassium conductance. J Physiol (Lond) 373:181–194
Block GA, Pulsinelli WA (1987) N-Methyl-D-Aspartate receptor antagonists: failure to prevent ischemia-induced selective neuronal damage. In: Raschle ME, Powers WJ (eds) Cerebrovascular diseases. Raven Press, NY, pp 37–44
Blomqvist P, Wieloch T (1985) Ischemic brain damage in rats following cardiac arrest using a long-term recovery model. J Cereb Blood Flow Metab 5:420–431
Blomqvist P, Mabe H, Ingvar M, Siesjo BK (1984) Models for studying long-term recovery following forebrain ischemia in the rat. 1. Circulatory and functional effects of 4-vessel occlusion. Acta Neurol Scand 69:376–384
Bolam JP, Wainer BH, Smith AD (1984) Characterization of cholinergic neurons in the rat neostriatum: a combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience 12:711–718
Buchan AMJ, Baimbridge KG (1988) Distribution and co-localization of calbindin D28K with VIP and neuropeptide Y but not somatostatin, galanin and substance P in the enteric nervous system of the rat. Peptides 9:333–338
Buchan A, Pulsinelli WA (1988) Neurotransmitters and neurotrophic factors in cerebral ischemia towards a molecular explanation for selective neuronal injury and a new rationale for stroke treatment. Int Symp Brain Resusc Jpn, pp 5–11
Buzsáki G, Czopf J, Kondakor I, Kellenyi L (1986) Laminar distribution of hippocampal rhythmic slow activity (RSA) in the behaving rat: current-source density analysis, effects of urethane and atropine. Brain Res 365:125–137
Buzsáki G, Freund TF, Bayardo F, Somogyi P (1989) Ischemia-induced changes in the electrical activity of the hippocampus. Exp Brain Res 78:268–278
Celio MR, Heizman CW (1981) Calcium-binding protein parvalbumin as a neuronal marker. Nature 293:300–302
Cowey A, Perry VH (1979) The projection of the temporal retina in the rat, studied by retrograde transport of horseradish peroxidase. Exp Brain Res 35:457–464
Crain BJ, Westerkam WD, Harrison AH, Nadler JV (1988) Selective neuronal death after transient forebrain ischemia in the Mongolian gerbil: a silver impregnation study. Neuroscience 27:387–402
DeLeo J, Toth L, Schubert P, Rudolphi K, Kreutzberg GW (1987) Ischemia-induced neuronal cell death, calcium accumulation, and glial response in the hippocampus of the Mongolian gerbil and protection by propentofylline (HWA 285) J Cereb Blood Flow Metab 7:745–751
Deshpande JK, Siesjo BK, Wieloch T (1987) Calcium accumulation and neuronal damage in the rat hippocampus following cerebral ischemia. J Cereb Blood Flow Metab 7:89–95
Dienel GA (1984) Regional accumulation of calcium in post-ischemic rat brain. J Neurochem 43:145–151
Francis A, Pulsinelli WA (1982) Response of GABAergic and cholinergic neurons to transient cerebral ischemia. Brain Res 243:271–278
Freund TF, Buzsaki G, Leon A, Somogyi P (1990) Hippocampal cell death following ischemia, effects of brain temperature and anaesthesia. Exp Neurol 108:251–260
Freund TF, Buzsáki G, Prohaska OJ, Leon A, Somogyi P (1989) Simultaneous recording of local electrical activity, partial oxygen tension and temperature in the rat hippocampus with a chamber-type microelectrode: effects of anaestesia, ischemia and epilepsy. Neuroscience 28:539–549
Gallyas F, Wolff JR, Bottcher H, Zaborszky L (1980) A reliable and sensitive method to localize terminal degeneration and lysosomes in the central nervous system. Stain Technol 55:299–306
Gerfen CR, Baimbridge KG, Miller JJ (1985) The neostriatal mosaic: Compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc Natl Acad Sci USA 82:8780–8784
Gill R, Foster AC, Woodruff GN (1987) Systemic administration of MK-801 protects against ischemia-induced hippocampal neurodegeneration in the gerbil. J Neurosci 7:3343–3349
Goldberg MP, Weiss JH, Pham P-C, Choi DW (1987) N-methyl-D-aspartate receptors mediate hypoxic neuronal injury in cortical culture. J Pharmacol Exp Ther 243:784–791
Hamon B, Heinemann U (1986) Eifects of GABA and bicuculline on N-methyl-D-aspartate- and quisqualate-induced reductions in extracellular free calcium in area CA1 of the hippocampal slice. Exp Brain Res 64:27–36
Heizmann CW (1984) Parvalbumin, an intracellular calcium-binding protein: distribution, properties and possible roles in mammalian cells. Experientia 40:910–921
Hossmann KA, Paschen W, Csiba L (1983) Relationship between calcium accumulation and recovery of cat brain after prolonged cerebral ischemia. J Cereb Blood Flow Metab 3:346–353
Ito U, Spatz M, Walker JT, Klatzo I (1975) Experimental cerebral ischemia in Mongolian gerbils. I. Light microscopic observations. Acta Neuropath (Berl) 32:209–223
Jarvis MF, Murphy DE, Williams M, Gerhardt SC, Boast CA (1988) The novel N-methyl-D-aspartate (NMDA) antagonist CGS 19755 prevents ischemia-induced reductions of adenosine A1, NMDA, and PCP receptors in gerbil brain. Synapse 2:577–584
Johansen FF, Jorgensen MB, Diemer NH (1987a) Ischemia-induced delayed neuronal death in the CA-1 hippocampus is dependent on intact glutamatergic innervation. In: Hicks TP, Lodge D, McLennan H (eds) Excitatory amino acid transmission. Alan R. Liss, New York, pp 245–248
Johansen FF, Lin C-T, Schousboe A, Wu J-Y (1989) Immunocytochemical investigation of L-glutamic acid decarboxylase in the rat hippocampal formation: the influence of transient cerebral ischemia. J Comp Neurol 281:40–53
Johansen FF, Zimmer J, Diemer NH (1987b) Early loss of somatostatin neurons in dentate hilus after cerebral ischemia in the rat precedes CA-1 pyramidal cell loss. Acta Neuropath (Berl) 73:110–114
Johansen FF, Diemer NH (1986) No loss of GABAergic inter-neurons in the rat hippocampal formation after 20 min of transient cerebral ischemia: an immunohistochemical investigation of L-glutamate decarboxylase. X. Internat Congr of Neuropathology Stockholm Convention Bureau, Stockholm, abstr 420, p 209
Jorgensen MB, Diemer NH (1982) Selective neuron loss after cerebral ischemia in the rat: possible role of transmitter glutamate. Acta Neurol Scand 66:536–546
Katsumaru H, Kosaka T, Heizmann CW, Hama K (1988) Immunocytochemical study of GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus. Exp Brain Res 72:347–362
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239:57–69
Kirino T, Tamura A, Sano K (1984) Delayed neuronal death in the rat hippocampus following transient forebrain ischemia. Acta Neuropathol (Berl) 64:139–147
Lynch G, Baudry M (1984) The biochemistry of memory: a new and specific hypothesis. Science 224:1057–1063
Mayer ML, MacDermott AB, Westbrook GL, Smith SJ, Barker JL (1987) Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci 7:3230–3244
Monaghan DT, Cotman CW (1985) Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci 5:2909–2919
Morrissey RL, Empson RN, Zolock DT, Bikle DD, Bucci TJ (1978) Intestinal response to 1,25-dihydroxycholecalciferol. II. A timed study of the intracellular localization of calcium-binding protein. Biochim Biophys Acta 538:34
Mudrick LA, Baimbridge KG (1989) Long-term structural changes in the rat hippocampal formation following cerebral ischemia. Brain Res 493:179–184
Nauta HJW, Butler AB, Jane AJ (1973) Some observations on axonal degeneration resulting from superficial lesions of the cerebral cortex. J Comp Neurol 150:349–360
Nitsch C, Scotti A, Sommacal A, Kalt G (1989) GABAergic hippo-campal neurons resistant to ischemia-induced neuronal death contain the Ca2+-binding protein parvalbumin. Neurosci Lett 105:263–268
Petito CK, Pulsinelli WA (1984) Sequential development of reversible and irreversible neuronal damage following cerebral ischemia. J Neuropath Exp Neurol 43:141–153
Prohaska O (1987) Thin-film micro-electrodes for in vivo electro-chemical analysis. In: Turner APF, Karube I, Wilson (eds) GS Biosensors, fundamentals and applications. Oxford University Press, Oxford, pp 377–389
Pulsinelli WA, Brierley JB (1979) A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke 10:267–272
Pulsinelli WA, Brierley JB, Plum F (1982a) Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol 11:491–498
Pulsinelli WA, Levy DE, Duffy TE (1982b) Regional cerebral blood flow and glucose metabolism following transient forebrain ischemia. Ann Neurol 11:499–509
Ross DT, Duhaime A-C (1989) Degeneration of neurons in the thalamic reticular nucleus following transient ischemia due to raised intracranial pressure: excitotoxic degeneration mediated via non-NMDA receptors? Brain Res 501:129–143
Rothman SM, Olney JW (1986) Glutamate and the patho-physiology of hypoxic-ischemic brain damage. Ann Neurol 19:105–111
Sakamoto N, Kogure K, Kato H, Ohtomo H (1986) Disturbed calcium homeostasis in the gerbil hippocampus following brief transient ischemia. Brain Res 364:372–376
Scharfman HE, Schwartzkroin PA (1989) Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science 246:257–260
Schiander M, Hoyer S, Frotscher M (1988) Glutamate decarboxylase-immunoreactive neurons in the aging rat hippocampus are more resistant to ischemia than CA1 pyramidal cells. 91:241–246
Schmidt-Kastner R, Hossmann K-A (1988) Distribution of ischemic neuronal damage in the dorsal hippocampus of rat. Acta Neuropathol 76:411–421
Schmidt-Kastner R, Paschen W, Grosse Ophoff B, Hossmann K-A (1989) A modified four-vessel occlusion model for inducing incomplete forebrain ischemia in rats. Stroke 20:938–946
Siesjo BK (1981) Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab 1:155–185
Siesjo BK, Bengtsson F (1989) Calcium fluxes, calcium antagonits, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J Cereb Blood Flow Metab 9:127–140
Siesjo BK, Wieloch T (1985) Cerebral metabolism in ischemia: neurochemical basis for therapy. Br J Anaesth 57:47–62
Simon RP, Swan JH, Griffiths T, Meldrum BS (1984) Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science 226:850–852
Sloviter RS (1989) Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific references to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol 280:183–196
Smith M-L, Auer RN, Siesjo BK (1984) The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathol (Berl) 64:319–332
Sofroniew MV, Eckenstein F, Thoenen H, Cuello AC (1982) Topography of choline acetyltransferase-containing neurons in the forebrain of the rat. Neurosci Lett 33:7–12
Strosznajder J (1980) Role of phospholipids in calcium accumulation in brain mitochondria from adult rat after ischemic anoxia and hypoxic hypoxia. Bull Acad Pol Sci Ser Biol 27:683–692
Swan JH, Evans MC, Meldrum BS (1988) Long-term development of selective neuronal loss and the mechanism of protection by 2-amino-7-phophonoheptanoate in a rat model of incomplete forebrain ischemia. J Cereb Blood Flow Metab 8:64–78
Thilmann R, Xie Y, Kleihues P, Kiessling M (1986) Persistent inhibition of protein synthesis precedes delayed neuronal death in postischemic gerbil hippocampus. Acta Neuropathol (Berl) 71:88–93
Todd NV, Picozzi P, Crockard HA, Russell RR (1986) Reperfusion after cerebral ischemia: influence of duration of ischemia. Stroke 17:460–466
Van Reempts J, Haseldonckx M, Van Deuren B, Wouters L, Borgers M (1986) Structural damage of the ischemic brain: involvement of calcium and effects of postischemic treatment with calcium entry blockers. Drug Dev Res 8:387–395
Wasterlain GG, Massarweh W, Sollas A, Rouk E, Sloviter RS (1988) Selective vulnerability of neurons to hypoxia-ischemia: role of calcium-binding protein. Soc Neurosci Abstr 14: p 1064
Yanagihara T, McCall JT (1982) Ionic shifts in cerebral ischemia. Life Sci 30:1921–1925
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Freund, T.F., Buzsáki, G., Leon, A. et al. Relationship of neuronal vulnerability and calcium binding protein immunoreactivity in ischemia. Exp Brain Res 83, 55–66 (1990). https://doi.org/10.1007/BF00232193
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00232193