Summary
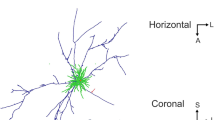
We used in vivo intracellular labeling with horseradish peroxidase in order to study the somadendritic morphology and axonal projections of rat entorhinal neurons. The cells responded to hippocampal stimulation with inhibitory postsynaptic potentials, and thus likely received direct or indirect hippocampal input. All cells (n = 24) showed extensive dendritic domains that extended in some cases for more than 1 mm. The dendrites of layer II neurons were largely restricted to layers I and II or layers I–III, while the dendrites of deeper cells could extend through all cortical layers. Computed 3D rotations showed that the basilar dendrites of deep pyramids extended roughly parallel to the cortical layering, and that they were mostly confined to the layer containing the soma and layers immediately adjacent. Total dendritic lengths averaged 9.8 mm ± 3.8 (SD), and ranged from 5 mm to more than 18 mm. Axonal processes could be visualized in 21 cells. Most of these showed axonal branching within the entorhinal cortex, sometimes extensive. Efferent axonal domains were reconstructed in detail in 3 layer II stellate cells. All 3 projected axons across the subicular complex to the dentate gyrus. One of these cells showed an extensive net-like axonal domain that also projected to several other structures, including the hippocampus proper, subicular complex, and the amygdalo-piriform transition area. The axons of layer III and IV cells projected to the angular bundle, where they continued in a rostral direction. In contrast to the layer II, III and IV cells, no efferent axonal branches leaving the entorhinal cortex could be visualized in 5 layer V neurons. The data indicate that entorhinal neurons can integrate input from a considerable volume of entorhinal cortex by virtue of their extensive dendritic domains, and provide a further basis for specifying the layers in which cells receive synaptic input. The extensive axonal branching pattern seen in most of the cells would support divergent propagation of their activity.
Similar content being viewed by others
Abbreviations
- AB:
-
angular bundle; Axes of the entorhinal cortex
- R:
-
radial axis of the Ent in the horizontal plane
- T:
-
tangential axis, perpendicular to R in the horizontal plane
- D/V:
-
dorsal-ventral axis
- DG:
-
dentate gyrus; Ent, entorhinal cortex;
- H:
-
distance in mm above the interaural line
- HiF:
-
hippocampal fissure
- HRP:
-
horseradish peroxidase
- IPSP:
-
inhibitory postsynaptic potential
- S:
-
subiculum
References
Adams JC (1981) Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem 29:775
Alonso A, Köhler C (1984) A study of the reciprocal connections between the septum and the entorhinal area using anterograde and retrograde axonal transport methods in the rat brain. J Comp Neurol 225:327–343
Alonso A, Llinás RR (1989) Subthreshold Na+-dependent thetalike rhythmicity in stellate cells of entorhinal cortex layer II. Nature 342:175–177
Barnes CA, McNaughton BL, Mizumori SJY, Leonard BW, Lin L-H (1989) Compact spatial representations are formed in hippocampus but not passed back to cortex. Soc Neurosci Abstr 15:776
Beckstead RM (1978) Afferent connections of the entorhinal area in the rat as demonstrated by retrograde cell-labeling with horseradish peroxidase. Brain Res 152:249–264
Brown R, Colter N, Corsellis AN, Crow TJ, Frith CD, Jagoe R, Johnstone EC, Marsh L (1986) Postmortem evidence of structural brain changes in schizophrenia. Arch Gen Psychiatry 43:36–42
Deadwyler SA, West JR, Cotman CW, Lynch G (1975) Physiological studies of the reciprocal connections between the hippocampus and entorhinal cortex. Exp Neurol 49:35–57
Falkai P, Bogerts B, Rozumek M (1988) Limbic pathology in schizophrenia: the entorhinal region-a morphometric study. Biol Psychiatry 24:515–521
Finch DM, Wong EE, Derian AL, Babb TL (1986a) Neurophysiology of limbic system pathways in the rat: projections from the subicular complex and hippocampus to the entorhinal cortex. Brain Res 397:205–213
Finch DM, Wong EE, Derian EL, Chen X-H, Nowlin-Finch NL, Brothers LA (1986b) Neurophysiology of limbic system pathways in the rat: projections from the amygdala to the entorhinal cortex. Brain Res 370:273–284
Finch DM, Tan AM, Isokawa-Akesson M (1988) Feedforward inhibition of the rat entorhinal cortex and subicular complex. J Neurosci 8: 2213–2226
Friauf E (1986) Morphology of motoneurons in different subdivisions of the rat facial nucleus stained intracellularly with horseradish peroxidase. J Comp Neurol 253:231–241
Germroth P, Schwerdtfeger WK, Buhl EH (1989a) GABAergic neurons in the entorhinal cortex project to the hippocampus. Brain Res 494:187–192
Germroth P, Schwerdtfeger WK, Buhl EH (1989b) Morphology of identified entorhinal neurons projecting to the hippocampus: a light microscopical study combining retrograde tracing and intracellular injection. Neurosci 30:683–691
Hjorth-Simonsen A (1972) Projection of the lateral part of the entorhinal area to the hippocampus and fascia dentata. J Comp Neurol 146:219–232
Hjorth-Simonsen A, Jeune B (1972) Origin and termination of the hippocampal perforant path studied by silver impregnation. J Comp Neurol 144:215–232
Itoh K, Konishi A, Nomura S, Mizuno N, Nakamura Y, Sugimoto T (1979) Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase: cobaltglucose oxidase method. Brain Res 175:341–346
Jakob H, Beckmann H (1986) Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm 65:303–326
Jeste DV, Lohr JB (1989) Hippocampal pathologic findings in schizophrenia. Arch Gen Psychiatry 46:1019–1024
Jones RSG, Heinemann U (1988) Synaptic and intrinsic responses of medial entorhinal cortical cells in normal and magnesium-free medium in vitro. J Neurophysiol 59:1476–1496
Kosel KC, Van Hoesen GW, Rosene DL (1982) Non-hippocampal cortical projections from the entorhinal cortex in the rat and rhesus monkey. Brain Res 244:201–213
Köhler C (1984) Morphological details of the projection from the presubiculum to the entorhinal area as shown with the novel PHA-L immunohistochemical tracing method in the rat. Neurosci Lett 45:285–290
Köhler C (1985) A projection from the deep layers of the entorhinal area to the hippocampal formation in the rat brain. Neurosci Lett 56:13–19
Köhler C (1986) Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J Comp Neurol 246:149–169
Köhler C (1988) Intrinsic connections of the retrohippocampal region in the rat brain. III. The lateral entorhinal area. J Comp Neurol 271:208–228
Larkman A and Mason A (1990) Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. I. Establishment of cell classes. J Neurosci 10:1407–1414
Light AR, Durkovic RG (1976) Horseradish peroxidase: an improvement in intracellular staining of single electrophysiologically characterized neurons. Exp Neurol 53:847–853
Lorente de Nó R (1933) Studies on the structure of the cerebral cortex. I. The area entorhinalis. J Psychol Neurol (Leipzig) 45:381–438
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, New York
Roberts GW, Bruton CJ (1990) Notes from the graveyard: neuropathology and schizophrenia. Neuropath Appl Neurobiol 16:3–16
Schwartzkroin PA, Mathers LH (1978) Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res 157:1–10
Shibata H (1988) A direct projection from the entorhinal cortex to the mammillary nuclei in the rat. Neurosci Lett 90:6–10
Shipley MT (1974) Presubiculum afferents to the entorhinal area and the Papez circuit. Brain Res 67:162–168
Snow PJ, Rose PK, Brown AG (1976) Tracing axons and axon collaterals of spinal neurons using intracellular injection of horseradish peroxidase. Science 191:312–313
Steward O (1976) Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol 167:285–314
Steward O, Scoville SA (1976) Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol 169:347–370
Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR (1990) Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med 322:789–794
Swanson LW, Köhler C (1986) Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. J Neurosci 6:3010–3023
Sørensen KE (1985a) Projections of the entorhinal area to the striatum, nucleus accumbens, and cerebral cortex in the guinea pig. J Comp Neurol 238:308–322
Sørensen KE (1985b) The connections of the hippocampal region. Acta Neurol Scand 550–560
Van Hoesen GW (1982) The parahippocampal gyrus: new observations regarding its cortical connections in the monkey. Trends Neurosci 5:345–350
White TD, Tan AM, Finch DM (1990) Functional reciprocal connections of the rat entorhinal cortex and subicular complex with the medial frontal cortex: an in vivo intracellular study. Brain Res 533:95–106
Witter MP, Groenewegen HJ, Lopes da Suva FH, Lohman AHM (1989) Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol 33:161–254
Wouterlood FG, Nederlof J (1983) Terminations of olfactory afferents on layer II and III neurons in the entorhinal area: degeneration-Golgi-electron microscopic study in the rat. Neurosci Lett 36:105–110
Wyss JM (1981) An autoradiographic study of the efferent connections of the entorhinal cortex in the rat. J Comp Neurol 199:495–512
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lingenhöhl, K., Finch, D.M. Morphological characterization of rat entorhinal neurons in vivo: soma-dendritic structure and axonal domains. Exp Brain Res 84, 57–74 (1991). https://doi.org/10.1007/BF00231762
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00231762