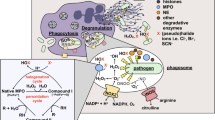
Summary
Polymorphonuclear leukocytes (PMN) or neutrophils have multiple systems available for killing ingested bacteria. Nearly each of these incorporates H2O2 indicating the essential nature of this reactive oxygen intermediate for microbicidal activity. Following ingestion of bacteria by PMN, H2O2 is formed by the respiratory burst which consumes O2 and generates H2O2 from O2−. H2O2 is deposited intracellularly near bacteria within phagocytic vacuoles where it can react with the MPO-H2O2-halide system to form toxic hyperchlorous acid (HOCl) and/or possibly singlet oxygen (1O2). H2O2 can also react with O2− and/or iron (Fe++) from lactoferrin or bacteria to form the highly toxic hydroxyl radical (1OH). These mechanisms appear important since deficiencies of H2O2 production, myeloperoxidase or lactoferrin frequently increases their owner's susceptibility to infection. In particular, examination of PMN from infection prone patients with chronic granulomatous disease (CGD) most clearly demonstrates the importance of H2O2 in killing of bacteria. CGD PMN lack the capacity to effectively generate H2O2 and subsequently have impaired ability to kill catalase positive (H2O2 producing) but not catalase negative (not H2O2 producing) bacteria. PMN also have catalase and glutathione peroxidase systems in their cytoplasms to protect themselves from the toxicity of H2O2. Finally, while H2O2 is critical for host defense, it can also be released extracellularly and thereby play a significant role in PMN mediated tissue injury.
Similar content being viewed by others
References
Keller, H. U., Hess, M. W. and Cottier, H., 1975. Semin. Hematol. 12: 47–57.
Zigmond, S. H., 1974. Nature 249: 450–452.
Stossel, T. P., 1975. Semin. Hematol. 12: 83–116.
Zucker-Franklin, D., 1964. J. Exp. Med. 120: 569–576.
Karnovsky, M. L., 1968. Semin. Hematol. 5: 156–165.
Repine, J. E., White, J. G., Clawson, C. C. et al., 1974. J. Clin. Invest. 54: 83–90.
Baldridge, C. W. and Gerard, R. W., 1933. Am. J. Physiol. 103: 235–235.
Iyer, G. Y. N., Islam, M. F. and Quastel, J. H., 1961. Nature 192: 535–541.
Sbarra, A. J. and Karnovsky, M. L., 1959. J. Biol. Chem. 234: 1355–1362.
Root, R. K. and Metcalf, J. A., 1977. J. Clin. Invest. 60: 1266–1279.
Root, R. K., Metcalf, J. A., Oshino, N. et al., 1975. J. Clin. Invest. 55: 945–955.
Goldstein, I. M., Cerquiera, M., Lind, S. et al., 1977. J. Clin. Invest. 59: 249–254.
Babior, B. M., Kipnes, R. S. and Curnutte, J. T., 1973. J. Clin. Invest. 52: 741–744.
Drath, D. B. and Karnovsky, M. L., 1975. J. Exp. Med. 141: 257–262.
McCord, J. M. and Fridovich, I., 1969. J. Biol. Chem. 244: 6049–6055.
Babior, B. M., Curnutte, J. T. and McMurrich, B. J., 1976. J. Clin. Invest. 58: 989–996.
Klebanoff, S. J., 1968. J. Bacteriol. 95: 2131–2138.
Klebanoff, S. J., 1967. J. Exp. Med. 126: 1063–1078.
McRipley, R. J. and Sbarra, A. J., 1967. J. Bacteriol. 94: 1425–1430.
Quie, P. G., White, J. G., Holmes, B. and Good, R. A., 1967. J. Clin. Invest. 46: 668–679.
Mandell, G. L. and Hook, E. W., 1969. J. Bacteriol. 100: 531–532.
Kaplan, E. L., Laxdal, T. and Quie, P. G., 1968. Pediatrics 41: 591–599.
Baehner, R. L., Nathan, D. G. and Karnovsky, M. L., 1970. J. Clin. Invest. 49: 865–870.
Johnston, R. B. Jr. and Baehner, R. L., 1970. Blood. 35: 350–355.
Root, R. K. Clin. Res. 22: 452A.
Klebanoff, S. J., 1967. J. Clin. Invest. 46: 1078.
Klebanoff, S. J. and Rosen, H., 1979. In: Oxygen Free Radicals and Tissue Damage. Excerpta Medica, Oxford, New York.
Bainton, D. F. and Farquhar, M. G., 1968. J. Cell. Biol. 39: 299–317.
Lehrer, R. I. and Cline, M. J., 1969. J. Clin. Invest. 48: 1478–1488.
Lehrer, R. I., Hanifen, J. and Cline, M. J., 1969. Nature 223: 78–79.
Klebanoff, S. J., 1970. Science 169: 1095–1097.
Rosen, H. and Klebanoff, S. J., 1977. J. Biol. Chem. 252: 4803–4810.
Allen, R. C., Stjernholm, R. L. and Steele, R. H., 1972. Biochem. Biophys. Res. Commun. 47: 679–684.
Kearns, D. R., 1971. Chem. Rev. 71: 395–427.
Cheson, B. D., Christensen, R. L., Sperling, R. et al., 1976. J. Clin. Invest. 58: 789–796.
Anderson, B. R., Brendzel, A. M. and Lint, T. F., 1977. Infect. Immun. 17: 62–66.
Rosen, H. and Klebanoff, S. J., 1976. J. Clin. Invest. 58: 50–60.
Piatt, J. F., Cheema, A. S. and O'Brien, P. J., 1977. FEBS Letters 74: 251–254.
Krinksy, N. I., 1974. Science 186: 363–365.
Khan, A., 1970. Science 168: 476–477.
Khan, A., 1977. J. Am. Chem. Sec. 99: 370–371.
Kellogg, E. W., Tert, R. S. and Fridovich, I., 1975. J. Biol. Chem. 250: 8812–8817.
Krinksy, N. I., 1979. In: Singlet Oxygen. N.Y. Acad. Press.
Babior, B. M., 1978. J. Engl. J. Med. 298: 659–668.
Haber, F. and Willstatter, R., 1931. Berichte 64: 2844–2856.
Haber, F. and Weiss, J., 1932. Naturwissenschaften 20: 948–950.
Fridovich, I., 1970. J. Biol. Chem. 245: 4053–4057.
Gregory, E. M. and Fridovich, I.. 1974. J. Bacteriol. 117: 166–169.
Webb, L. S., Keele, B. B. Jr. and Johnston, R. B. Jr., 1974. Infect. Immun. 9: 1051–1056.
Beauchamp, C. and Fridovich, I., 1970. J. Biol. Chem. 245: 4641–4646.
Repine, J. E., Eaton, J. A., Anders, M. W. et al., 1979. J. Clin. Invest. 64: 1642–1651.
Fenton, H. J. H., 1893. J. Chem. Soc. 64: 899–904.
Fee, J. A. and Valentine, J. S., 1977. In: Superoxide and Superoxide Dismutases. London & N.Y. Acad. Press. pp. 19–60.
Fee, J. A., 1980. In: Metal Ion Activation of Dioxygen. J. Wiley & Sons, Inc. pp. 209–237.
Halliwell, B., 1976. Fed. Eur. Biochem. Soc. Lett. 72: 8–10.
McCord, J. M. and Day, E. D. Jr., 1978. Fed. Eur. Biochem. Soc. Lett. 86: 139–142.
Repine, J. E., Fox, R. B., Berger, E. M. and Harada, R. N., 1981. Infect. Immun. 32: 407–410.
Repine, J. E., Fox, R. B. and Berger, E. M., 1981. J. Biol. Chem. 256: 7094–7097.
Arnold, R. R., Brewer, M. and Gauthier, J. J., 1980. Infect. Immun. 28 (3): 893–898.
Breton-Gorius, J., Mason, D. Y., Buriot, D. et al., 1980. Am. J. Path. 99 (2): 413–428.
Weening, R. S., Roos, D., Van Schaik, M., Voetman, A. A., DeBoer, M. and Loos, J. A., 1977. In: Movement, Metabolism and Bactericidal Mechanisms of Phagocytes, Piccin Medical Books, Padua, pp. 277–283.
Holmes, B., Park, B. H., Malawista, S. E., Quie, P. G., Nelson, D. L. and Good, R. A., 1970. N. Engl. J. Med. 283: 217–221.
Shasby, D. M., Van Benthuysen, K. M., Tate, R. M. et al., 1982. Amer. Rev. Resp. Dis. 125: 443–447.
Tate, R. M., Van Benthuysen, K. M., Shasby, D. M. et al., 1982. Amer. Rev. Resp. Dis. (in press).
Nathan, C. F. and Cohn, Z. A., 1981. J. Exp. Med. 154: 1539–1553.
De Filippi, L. J., Ballou, D. P. and Hultquist, D. E., 1979. J. Biol. Chem. 254: 6917–6923.
Hoffmann, M. E. and Meneghini, R., 1979. Photochem. and Photobiol. 30: 151–155.
Hoffmann, M. E. and Meneghini, R., 1979. Photochem. and Photobiol. 29: 299–303.
Nathan, C. F., Silverstein, S. C., Builner, L. H. et al., 1979. J. Exp. Med. 149: 100–113.
Clark, R. A. and Klebanoff, S. J., 1980. J. Immunol. 124: 399–405.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Clifford, D.P., Repine, J.E. Hydrogen peroxide mediated killing of bacteria. Mol Cell Biochem 49, 143–149 (1982). https://doi.org/10.1007/BF00231175
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00231175