Summary
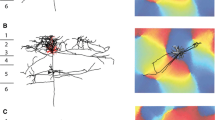
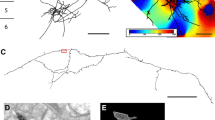
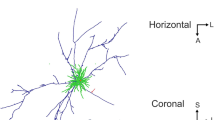
The morphology of single neurons in area 3b of cat primary somatosensory (SI) cortex was examined after horseradish peroxidase (HRP) injections. Neurons were labeled either by intracellular injection of HRP following intracellular recording or by small extracellular iontophoretic HRP injections. Both pyramidal and nonpyramidal neurons were labeled and reconstructed from serial sections. Their axons had local, interlaminar and interareal patterns of termination. Most neurons formed local axonal fields around their cell bodies and dendrites. Pyramidal neurons in cortical layer IV sent axons up into layers II and III, neurons in layers II and III sent axons down to layer V, and layer V neurons sent axons to layer VI as well as back to the upper layers. Layer VI neurons sent axons back to the upper cortical layers in a unique bowl-shaped pattern. The horizontal distribution of axons of pyramidal cells in layer III was extremely widespread. Axons of layer III neurons in area 3b terminated within 3b and area 1, but not in other areas of SI. Layer III neurons in area 1 distributed axon collaterals to all fields of SI as well as projecting a main axon to motor cortex. In general, the axon collaterals of area 3b pyramidal cells outside layer III remained confined to area 3b. Most of the nonpyramidal neurons labeled were basket cells in layers III and VI. These neurons formed dense axonal fields around their cell bodies, and none of their axons could be followed into the underlying white matter. The results of the present study demonstrate that area 3b somatosensory cortical neurons and their axons are vertically organized in a manner similar to that reported for other sensory cortical areas. They also show that widespread horizontal connections are formed by pyramidal neurons of layer III, and that these horizontal axons can travel for great distances in the cortical grey matter.
Similar content being viewed by others
References
Adams JC, Warr WB (1976) Origins of axons in the cat's acoustic striae determined by injection of horseradish peroxidase into severed tracts. J Comp Neurol 170:107–121
Bolz J, Gilbert CD (1986) Generation of end-inhibition in the visual cortex via interlaminar connections. Nature 320:362–365
Brodmann K (1906) Beiträge zur histologischen Lokalisation der Großhirnrinde. V. Über den allgemeinen Bauplan des Cortex pallii bei den Mammalien und zwei homologe Rindenfelder im Besonderen: zugleich ein Beitrag zur Furchenlehre. J Psychol Neurol 6:275–400
DeFelipe J, Conley M, Jones EG (1986) Long-range focal collateralization of axons arising from corticocortical cells in monkey sensory-motor cortex. J Neurosci 6:3749–3766
Dykes RW, Landry P, Hicks TP, Diadori P, Metherate R (1987) Specificity of connections in the ventroposterior nuclei of the thalamus. Prog Neurobiol 30:87–103
Ferster D, Lindstrom S (1983) An intracellular analysis of geniculo-cortical connectivity in area 17 of the cat. J Physiol (Lond) 342:181–215
Friedman DP, Jones EG (1981) Thalamic input to areas 3a and 2 in monkeys. J Neurophysiol 45:59–85
Gardner EP, Spencer WA (1972) Sensory funneling. II. Cortical neuronal representation of patterned cutaneous stimuli. J Neurophysiol 35:954–977
Gilbert CD (1983) Microcircuitry of the visual cortex. Annu Rev Neurosci 6:217–247
Gilbert GD, Wiesel TN (1979) Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature 280:120–125
Hand PJ, Morrison AR (1970) Thalamocortical projections from the ventrobasal complex to somatic sensory areas I and II. Exp Neurol 26:291–308
Hassler R, Muhs-Clement K (1964) Architektonischer Aufbau des sensorimotorischen und parietalen Cortex der Katze. J Hirnforsch 6:377–420
Hyvärinen J, Poranen A (1978a) Movement-sensitive and direction and orientation-selective cutaneous receptive fields in the hand area of the post-central gyrus in monkeys. J Physiol (Lond) 283:523–537
Hyvärinen J, Poranen A (1978b) Receptive field integration and submodality convergence in the hand area of the postcentral gyrus of the alert monkey. J Physiol (Lond) 283:539–556
Iwamura Y, Tanaka M (1978) Functional organization of receptive fields in the cat somatosensory cortex. I. Integration within the coronal region. Brain Res 151:49–60
Iwamura Y, Tanaka M, Sakamoto M, Hikosaka O (1983) Con- verging patterns of finger representation and complex response properties of neurons in area 1 of the first somatosensory cortex of the conscious monkey. Exp Brain Res 51:327–337
Iwamura Y, Tanaka M, Sakamoto M, Hikosaka O (1985) Verti- cal neuronal arrays in the postcentral gyrus signaling active touch: a receptive field study in the conscious monkey. Exp Brain Res 58:412–420
Jones EG, Friedman DP (1982) Projection pattern of functional components of thalamic ventrobasal complex on monkey somatosensory cortex. J Neurophysiol 48:521–544
Jones EG, Porter R (1980) What is area 3a? Brain Res Rev 2:1–43
Jones EG, Powell TPS (1969a) Connexions of the somatic sensory cortex of the rhesus monkey. I. Ipsilateral cortical connexions. Brain 92:477–502
Jones EG, Powell TPS (1969b) The cortical projection of the ventroposterior nucleus of the thalamus in the cat. Brain Res 13:298–318
Jones EG, Coulter JD, Hendry SHC (1978) Intracortical connectivity of architectonic fields in the somatic, motor, and parietal cortex of monkeys. J Comp Neurol 181:291–348
Juliano SL, Whitsel BL, Tommerdahl M, Cheema SS (1989) Determinants of patchy metabolic labeling in the somatosensory cortex of cats: a possible role for intrinsic inhibitory circuitry. J Neurosci 9:1–12
Kaas JH (1983) What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol Rev 63:206–231
Kaas JH, Nelson RJ, Sur M, Lin C-S, Merzenich MM (1979) Multiple representations of the body within the primary somatosensory cortex of primates. Science 204:521–523
Kisvardy ZF, Martin KAC, Freund TF, Magloczky ZS, Whitteridge D, Somogyi P (1986) Synaptic targets of HRP-filled layer III pyramidal cells in the cat striate cortex. Exp Brain Res 64:541–552
Laskin SE, Spencer WA (1979) Cutaneous masking. II. Geometry of excitatory and inhibitory receptive fields of single units in somatosensory cortex of the cat. J Neurophysiol 42:1061–1082
Lund JS, Henry GH, MacQueen CL, Harvey AR (1979) Anatomical organization of the primary visual cortex (area 17) of the cat: a comparison with area 17 of the macaque monkey. J Comp Neurol 184:599–618
Martin KAC, Whitteridge D (1984) Form, function and intra- cortical projections of spiny neurones in the striate visual cortex of the cat. J Physiol (Lond) 353:463–504
McKenna TM, Light AR, Whitsel BL (1984) Neurons with unusual response and receptive-field properties in upper laminae of cat SI cortex. J Neurophysiol 51:1055–1076
Mountcastle VB (1957) Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol 20:408–434
Mountcastle VB, Powell TPS (1959) Neural mechanisms subserving cutaneous sensibility, with special reference to the role of afferent inhibition in sensory perception and discrimination. Bull Johns Hopkins Hosp 105:201–232
Nauta HJW, Butler AB, Jane JA (1973) Some observations on axonal degeneration resulting from superficial lesions of the cerebral cortex. J Comp Neurol 150:349–360
Powell TPS, Mountcastle VB (1959) Some aspects of the functional organization of the cortex of the postcentral gyrus of the monkey: a correlation of findings obtained in a single unit analysis with cytoarchitecture. Bull Johns Hopkins Hosp 105:133–162
Shanks MF, Pearson RC, Powell TPS (1985) The ipsilateral corticocortical connections between the cytoarchitectonic subdivisions of primary somatic sensory cortex in the monkey. Brain Res Rev 9:67–88
Schwark HD, Hirai T, Jones EG (1986) Laminar organization of area 3b of cat somatosensory cortex. Soc Neurosci Abstr 12:1430
Sur M, Garraghty PE, Brace CJ (1985) Somatosensory cortex in macaque monkeys: laminar differences in receptive field size in areas 3b and 1. Brain Res 342:391–395
Vogt BA, Pandya DN (1977) Cortico-cortical connections of somatic sensory cortex (areas 3, 1, and 2) in the rhesus monkey. J Comp Neurol 177:179–191
Vogt C, Vogt O (1919) Allgemeinere Ergebnisse unserer Hirnforschung. J Psychol Neurol 25:277–462
Yamamoto T, Samejima A, Oka H (1987) Morphology of layer V pyramidal neurons in the cat somatosensory cortex: an intracellular HRP study. Brain Res 437:369–374
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schwark, H.D., Jones, E.G. The distribution of intrinsic cortical axons in area 3b of cat primary somatosensory cortex. Exp Brain Res 78, 501–513 (1989). https://doi.org/10.1007/BF00230238
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230238