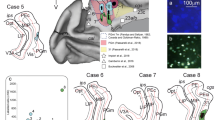
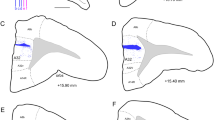
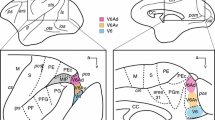
Abstract
Prefrontostriatal and prefrontothalamic connections in rhesus monkeys have been shown to be organized in a topographic manner. These projections originate largely from infragranular layers V and VI. To examine whether the striatal and thalamic connections from the prefrontal cortex arise from separate neuronal populations or are collateralized, two different fluorescent retrograde tracers (diamidino yellow and fast blue) were injected into topographically similar regions of the head of the caudate nucleus and the mediodorsal nucleus in the same animal. The results show that although prefrontostriatal and prefrontothalamic projections arise from similar topographic regions, their laminar origins are distinctive. The connections to the head of the caudate nucleus originate mainly from layer Va, to a lesser extent from layer Vb, with a minor contribution from layers III and VI. In contrast, the projections to the mediodorsal nucleus emanate largely from layer VI, and also from layer Vb. Only occasional double-labeled neurons were observed, indicating that prefrontostriatal and prefrontothalamic connections originate from separate neuronal populations. The differential laminar distributions of neurons projecting to the head of the caudate nucleus and the mediodorsal nucleus suggest that these structures may receive independent types of information from the prefrontal cortex.
Similar content being viewed by others
References
Aggleton JP, Mishkin M (1983) Visual recognition impairment following medial thalamic lesions in monkeys. Neuropsychologia 21:189–197
Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:266–271
Alexander GE, Fuster JM (1973) Effects of cooling prefrontal cortex on cell firing in the nucleus medialis dorsalis. Brain Res 61:93–105
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Arikuni T, Kubota K (1986) The organization of prefrontocaudate projections and their laminar origin in the macaque monkey: a retrograde study using HRP-gel. J Comp Neurol 244:492–510
Arikuni T, Sakai M, Kubota K (1983) Columnar aggregation of prefrontal and anterior cingulate cortical cells projecting to the thalamic mediodorsal nucleus in the monkey. J Comp Neurol 220:116–125
Azuma M, Nakayama H, Sasaki Y, Suzuki H (1988) Relation between visual input and motor outflow for eye movements in monkey frontal eye field. Behav Brain Res 27:93–98
Barbas H, Pandya DN (1989) Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol 286:353–375
Bauer RH, Fuster JM (1976) Delayed-matching and delayed-response deficit from cooling dorsolateral prefrontal cortex in monkeys. J Comp Physiol Psychol 90:293–302
Benjamin RM, Burton H (1968) Projection of taste nerve afferents to anterior opercular-insular cortex in squirrel monkey (Saimiri sciureus). Brain Res 7:221–231
Benjamin RM, Jackson JC (1974) Unit discharge in the mediodorsal nucleus of the squirrel monkey evoked by electrical stimulation of the olfactory bulb. Brain Res 75:181–191
Butter CM, Snyder DR (1972) Alterations in aversive and aggressive behaviors following orbital frontal lesions in rhesus monkeys. Acta Neurobiol Exp 32:525–565
Butter CM, Mishkin M, Mirsky AF (1968) Emotional responses toward humans in monkeys with selective frontal lesions. Physiol Behav 3:213–215
Cavada C, Goldman-Rakic PS (1991) Topographic segregation of corticostriatal projections from posterior parietal subdivisions in the macaque monkey. Neuroscience 42:683–696
Collin NG, Cowey A, Latto R, Marzi C (1982) The role of frontal eye-fields and superior colliculi in visual search and non-visual search in rhesus monkeys. Behav Brain Res 4:177–193
Dassonville P, Schlag J, Schlag-Rey M (1992) The frontal eye field provides the goal of saccadic eye movement. Exp Brain Res 89:300–310
DiPellegrino G, Wise SP (1991) A neurophysiological comparison of three distinct regions of the primate frontal lobe. Brain 114:951–978
Divac I (1972) Neostriatum and functions of prefrontal cortex. Acta Neurobiol Exp 32:461–477
Ferino F, Thierry AM, Saffroy M, Glowinski J (1987) Interhemispheric and subcortical collaterals of medial prefrontal cortical neurons in the rat. Brain Res 417:257–266
Fisher RS, Boylan MK, Hull CD, Buchwald NA, Levine MS (1986) Branched projections of cat sensorimotor cortex: multiple retrograde labeling via commissural corticocortical, decussated corticostriatal and undecussated corticostriatal axons. Brain Res 384:395–400
Funahashi S, Bruce CJ, Goldman-Rakic PS (1991) Neuronal activity related to saccadic eye movements in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 65:1464–1483
Funahashi S, Bruce CJ, Goldman-Rakic PS (1993) Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas”. J Neurosci 13:1479–1497
Fuster JM, Alexander GE (1973) Firing changes in cells of the nucleus medialis dorsalis associated with delayed response behavior. Brain Res 61:79–91
Gaffan D, Harrison S (1989) A comparison of the effects of fornix transection and sulcus principalis ablation upon spatial learning by monkeys. Behav Brain Res 31:207–220
Gaffan D, Murray EA (1990) Amygdalar interaction with the mediodorsal nucleus of the thalamus and the ventromedial prefrontal cortex in stimulus-reward associative learning in the monkey. J Neurosci 10:3479–3493
Ganchrow D, Erickson RP (1972) Thalamocortical relations in gustation. Brain Res 36:289–305
Giguere M, Goldman-Rakic PS (1988) Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol 277:195–213
Goldberg ME, Bruce CJ (1985) Cerebral cortical activity associated with the orientation of visual attention in the rhesus monkey. Vision Res 25:471–481
Hallowitz RA, MacLean PD (1977) Effects of vagal volleys on units of intralaminar and juxtalaminar thalamic nuclei in monkeys. Brain Res 130:271–286
Hikosaka O, Sakamoto M, Usui S (1989a) Functional properties of monkey caudate neurons. I. Activities related to saccadic eye movements. J Neurophysiol 61:780–798
Hikosaka O, Sakamoto M, Usui S (1989b) Functional properties of monkey caudate neurons. II. Visual and auditory responses. J Neurophysiol 61:799–813
Hikosaka O, Sakamoto M, Usui S (1989c) Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J Neurophysiol 61:814–832
Huerta MF, Kaas JH (1990) Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J Comp Neurol 293:299–330
Isseroff A, Rosvold HE, Galkin TW, Goldman-Rakic PS (1982) Spatial memory impairments following damage to the mediodorsal nucleus of the thalamus in rhesus monkeys. Brain Res 232:97–113
Iversen SD, Mishkin M (1970) Perseverative interference in monkeys following selective lesions of the inferior prefrontal covexity. Exp Brain Res 11:376–386
Iversen SD, Mishkin M (1973) Comparison of superior temporal and inferior prefrontal lesions on auditory and non-auditory tasks in rhesus monkeys. Brain Res 55:355–367
Jones EG (1985) The thalamus. Plenum, New York
Jones EG, Burton H (1976) Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol 168:197–248
Jones EG, Coulter JD, Burton H, Porter R (1977) Cells of origin and terminaldistribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol 173:53–80
Kojima S, Goldman-Rakic PS (1984) Functional analysis of spatially discriminative neurons in prefrontal cortex of rhesus monkey. Brain Res 291:229–240
Kowalska DM, Bachevalier J, Mishkin M (1991) The role of the inferior prefrontal convexity in performance of delayed non-matching-to-sample. Neuropsychologia 29:583–600
Kusama T, Fujioka M, Miyakawa Y, Fujii M (1985) Connections of the fronto-parietal operculum with the posterior ventral thalamic nucleus, especially its medial nucleus, in monkeys. J Hirnforsch 26:317–331
Lawler KA, Cowey A (1987) On the role of posterior parietal and prefrontal cortex in visuospatial perception and attention. Exp Brain Res 65:695–698
Lynch JC (1987) Frontal eye field lesions in monkeys disrupt visual pursuit. Exp Brain Res 68:437–441
MacAvoy MG, Gottlieb JP, Bruce CJ (1991) Smooth-pursuit eye movement representation in the primate frontal eye field. Cereb Cortex 1:95–102
Motokizawa F (1974) Olfactory input to the thalamus: electrophysiological evidence. Brain Res 67:334–337
Nakano Y, Oomura Y, Nishino H, Aou S, Yamamoto T, Nemoto S (1984) Neuronal activity in the medial orbitofrontal cortex of the behaving monkey: modulation by glucose and satiety. Brain Res Bull 12:381–385
Niki H, Watanabe M (1976) Prefrontal unit activity and delayed response: relation to cue location versus direction of response. Brain Res 105:79–88
Nishino H, Ono T, Sasaki K, Fukuda M, Muramoto KI (1984) Caudate unit activity during operant feeding behavior in monkeys and modulation by cooling prefrontal cortex. Behav Brain Res 11:21–33
Ogawa H, Ito S-I, Nomura T (1989) Oral cavity representation at the frontal operculum of macaque monkeys. Neurosci Res 6:283–298
Parthasarathy HB, Schall JD, Graybiel AM (1992) Distributed but convergent ordering of corticostriatal projections: analysis of the frontal eye field and the supplementary eye field in the macaque monkey. J Neurosci 12:4468–4488
Passingham RE (1975) Delayed matching after selective prefrontal lesions in monkeys (Macaca mulatta). Brain Res 92:89–102
Passingham RE (1985) Memory of monkeys (Macaca mulatta) with lesions in prefrontal cortex. Behav Neurosci 99:3–21
Plata-Salamán CR, Scott TR (1992) Taste neurons in the cortex of the alert cynomolgus monkey. Brain Res Bull 28:333–336
Pritchard TC, Hamilton RB, Morse JR, Norgren R (1986) Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol 244:213–228
Quintana J, Yajeya J, Fuster JM (1988) Prefrontal representation of stimulus attributes during delay tasks. I. Unit activity in cross-temporal integration of sensory and sensory-motor information. Brain Res 474:211–221
Raleigh MJ, Steklis HD (1981) Effects of orbitofrontal and temporal neocortical lesions on the affiliative behavior of vervet monkeys (Cercopithecus aethiops sabaeus). Exp Neurol 73:378–389
Rizzolatti G, Scandolara C, Matelli M, Gentilucci M (1981) Afferent properties of periarcuate neurons in macaque monkeys. II. Visual responses. Behav Brain Res 2:147–163
Roberts TS, Akert K (1963) Insular and opercular cortex and its thalamic projection in Macaca mulatta. Schweiz Arch Neurol Psychiatr 92:1–43
Rockland KR, Pandya DN (1979) Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res 179:3–20
Rolls ET, Thorpe SJ, Maddison SP (1983) Responses of striatal neurons in the behaving monkey. 1. Head of the caudate nucleus. Behav Brain Res 7:179–210
Rolls ET, Scott TR, Sienkiewicz ZJ, Yaxley S (1988) The responsiveness of neurones in the frontal opercular gustatory cortex of the macaque monkey is independent of hunger. J Physiol (Lond) 397:1–12
Rolls ET, Yaxley S, Sienkiewicz ZJ (1990) Gustatory responses of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. J Neurophysiol 64:1055–1066
Rosene DL, Roy NJ, Davis BJ (1986) A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem 34:1301–1315
Rosenkilde CE, Bauer RH, Fuster JM (1981) Single cell activity in ventral prefrontal cortex of behaving monkeys. Brain Res 209:375–394
Rosvold HE (1972) The frontal lobe system: Cortical-subcortical interrelationships. Acta Neurobiol Exp 32:439–460
Rubinstein EH, Delgado JMR (1963) Inhibition induced by forebrain stimulation in the monkey. Am J Physiol 205:941–948
Russchen FT, Amaral DG, Price JL (1987) The afferent input to the magnocellular division of the mediodorsal thalamic nucleus in the monkey, Macaca fascicularis. J Comp Neurol 256:175–210
Saint-Cyr JA, Ungerleider LG, Desimone R (1990) Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. J Comp Neurol 298:129–156
Schall JD (1991) Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: comparison with supplementary eye fields. J Neurophysiol 66:559–579
Schlag J, Schlag-Rey M (1984) Visuomotor functions of central thalamus in monkey. II. Unit activity related to visual events, targeting, and fixation. J Neurophysiol 51:1175–1195
Schlag J, Schlag-Rey M (1986) Role of the central thalamus in gaze control. In: Freund HJ, Büttner U, Cohen B, Noth J (eds) The oculomotor and skeletalmotor system: differences and similarities. Prog Brain Res 64:191–201
Schlag-Rey M, Schlag J (1984) Visuomotor functions of central thalamus in monkey. I. Unit activity related to spontaneous eye movements. J Neurophysiol 51:1149–1174
Schultz W, Apicella P, Scarnati E, Ljungberg T (1992) Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci 12:4595–4610
Scott TR, Yaxley S, Sienkiewicz ZJ, Rolls ET (1986) Gustatory responses in the frontal opercular cortex of the alert cynomolgus monkey. J Neurophysiol 56:876–890
Selemon LD, Goldman-Rakic PS (1985) Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci 5:776–794
Selemon LD, Goldman-Rakic PS (1988) Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 8:4049–4068
Siwek DF, Pandya DN (1991) Prefrontal projections to the mediodorsal nucleus of the thalamus in the rhesus monkey. J Comp Neurol 31:509–524
Siwek DF, Pandya DN (1992) The differential laminar distribution of cells in prefrontal cortex projecting to the mediodorsal nucleus. Soc Neurosci Abstr 18:304
Spatz WB (1977) Topographically organized reciprocal connections between areas 17 and MT (visual area of superior temporal sulcus) in the marmoset Callithrix jacchus. Exp Brain Res 27:559–572
Stamm JS (1973) Functional dissociation between the inferior and arcuate segments of dorsolateral prefrontal cortex in the monkey. Neuropsychologia 11:181–190
Tanabe T, Yarita H, Iino M, Ooshima Y, Takagi SF (1975) Olfactory projection area in orbitofrontal cortex of the monkey. J Neurophysiol 38:1269–1283
Tanila H, Carlson S, Linnankoski I, Kahila H (1993) Regional distribution of functions in dorsolateral prefrontal cortex of the monkey. Behav Brain Res 53:63–72
Vaadia E, Benson DA, Heinz RD, Goldstein MH Jr (1986) Unit study of monkey frontal cortex: active localization of auditory and visual stimuli. J Neurophysiol 56:934–952
Van Essen DC, Maunsell JHR (1983) Hierarchical organization and functional streams in the visual cortex. Trends Neurosci 6:370–375
Walker AE (1940) A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol 73:59–86
Welch K, Stuteville P (1958) Experimental production of unilateral neglect in monkeys. Brain 81:341–347
Wilson FAW, Scalaidhe SPO, Goldman-Rakic PS (1993) Dissociation of object and spatial processing domains in the primate prefrontal cortex. Science 260:1955–1958
Wirth FP (1973) Insular-diencephalic connections in the macaque. J Comp Neurol 150:361–392
Yarita H, Iino M, Kogure S, Takagi SF (1980) A transthalamic olfactory pathway to orbitofrontal cortex in the monkey. J Neurophysiol 43:69–85
Yeterian EH, Pandya DN (1991) Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J Comp Neurol 312:43–67
Yeterian EH, Pandya DN (1992) Laminar origin of striatal and thalamic projections of the prefrontal cortex in rhesus monkeys. Soc Neurosci Abstr 18:305
Yeterian EH, Van Hoesen GW (1978) Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res 139:43–63
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yeterian, E.H., Pandya, D.N. Laminar origin of striatal and thalamic projections of the prefrontal cortex in rhesus monkeys. Exp Brain Res 99, 383–398 (1994). https://doi.org/10.1007/BF00228975
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228975