Summary
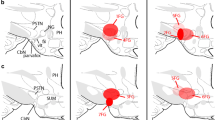
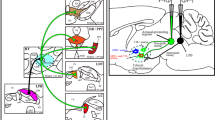
The descending projection of the hypothalamic paraventricular nucleus (PVN) to the A5 area was elucidated using a technique that combines retrograde labeling with horseradish peroxidase (HRP), anterograde labeling with PHA-L (Phaseolus vulgaris) leucoagglutinin and immunohistochemistry for dopamineβ-hydroxylase (DBH). Following an iontophoretic injection of PHA-L into the PVN, HRP was applied to the greater petrosal nerve. Frozen sections of the hypothalamus and the caudal pons were first treated according to a protocol for HRP histochemistry using tetramethylbenzidine with cobalt-enhanced diaminobenzidine, and then they were processed for displaying PHA-L, and then for DBH immunohistochemistry. PHA-L labeled fibers from the PVN were observed in a ventrolateral part of the pontine reticular formation corresponding to the A5 area, where they give rise to a dense network around the cells of origin of the greater petrosal nerve (GPN cells) and DBH-positive cells. Terminals or varicosities labeled with PHA-L were preferentially observed around the somata of GPN cells, suggesting direct contact. However, apparent contact between both elements was hardly ever observed. On the other hand, terminals or varicosities were occasionally observed in close relation to DBH positive cells. These results suggest that descending fibers of the PVN project more strongly to GPN cells than to DBH-positive cells. The relationship of this fiber pathway to control of the secretomotor or cardiovascular systems is discussed.
Similar content being viewed by others
References
Armstrong WE, Warach S, Hatton GI, McNeil TH (1980) Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience 5:1931–1958
Akmayev IG, Vikhreva OV, Konovalova LK (1981) The origin of the hypothalamic-vagal descending pathway: an experimental ultrastructural study. Brain Res 230:342–345
Byrum CE, Stornetta R, Guyenet PG (1984) Electrophysiological properties of spinally-projecting A5 noradrenergic neurons. Brain Res, 303:15–29
Byrum CE, Guyenet PG (1987) Afferent and efferent connections of the A5 noradrenergic cell group in the rat. J Comp Neurol 261:529–542
Conrad LCA, Pfaff DW (1976) Efferents from medial basal forebrain and hypothalamus in the rat. II. An autoradiographic study of the anterior hypothalamus. J Comp Neurol 169:221–262
Contreras RJ, Gomez MM, Norgren R (1980) Central origins of cranial nerve parasympathetic neurons in the rat. J Comp Neurol 190:373–394
Gerfen CR, Sawchenko PE (1984) An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res 290:219–238
Gold RM, Jones AP, Sawchenko P (1977) Paraventricular area: critical focus of a longitudinal neurocircuitry mediating food intake. Physiol Behav 18:1111–1119
Gray TS, O'Donohue TL, Magnuson DJ (1986) Neuropeptide Y innervation of amygdaloid and hypothalamic neurons that project to the dorsal vagal complex in rat. Peptides 7:341–349
Holstege G (1987) Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: an HRP and autoradiographic tracing study in the cat. J Comp Neurol 260:98–126
Hosoya Y, Matsushita M, Sugiura Y (1983) A direct hypothalamic projection to the superior salivatory nucleus neurons in the rat: a study using anterograde autoradiographic and retrograde HRP methods. Brain Res 266:329–333
Hosoya Y, Matsushita M, Sugiura Y (1984) Hypothalamic descending afferents to cells of origin of the greater petrosal nerve in the rat, as revealed by a combination of retrograde HRP and anterograde autoradiographic techniques. Brain Res 290:141–145
Leibowitz SF (1978) Paraventricular nucleus: a primary site mediating adrenergic stimulation of feeding and drinking. Pharmacol Biochem Behav 8:163–175
Luiten PGM, Ter Horst GJ, Karst H, Steffens AB (1985) The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res 329:374–378
Loewy AD, McKellar S, Saper CB (1979) Direct projections from the A5 catecholamine cell group to the intermediolateral cell column. Brain Res 174:309–314
Loewy AD, Marson L, Parkinson D, Perry MA, Sawyer WB (1986) Descending noradrenergic pathways involved in the A5 depressor response. Brain Res 386:313–324
Lovick TA, Coote JH (1988) Electrophysiological properties of paraventriculo-spinal neurons in the rat. Brain Res 454:123–130
Neil JJ, Loewy AD (1982) Decreases in blood pressure in response to L-glutamate microinjections in the A5 catecholamine cell group. Brain Res 241: 271–278
Rye DB, Saper CB, Wainer BH (1984) Stabilization of the tetramethylbenzidine (TMB) reaction product: application for retrograde and anterograde tracing, and combination with immunohistochemistry. J Histochem Cytochem 32:1145–1153
Saper CB, Loewy AD, Swanson LW, Cowan WM (1976) Direct hypothalamo-autonomic connections. Brain Res 117:305–312
Sawchenko PE, Gold RM, Leibowitz SF (1981) Evidence for vagal involvement in the eating elicited by adrenergic stimulation of the paraventricular nucleus. Brain Res 225:249–269
Swanson LW, Kuypers HGJM (1980) The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194:555–570
Swanson LW, Sawchenko PE, Wiegand SJ, Price JL (1980) Separate neurons in the paraventricular nucleus project to the median eminence and to the medulla or spinal cord. Brain Res 198:190–195
Ter Horst GJ, Luiten PGM, Kuipers F (1984) Descending pathways from hypothalamus to dorsal motor vagus and ambiguus nuclei in the rat. J Auton Nerv Syst 11:59–75
Westlund KN, Bowker RM, Ziegler MG, Coulter JD (1983) Noradrenergic projections to the spinal cord of the rat. Brain Res 263:15–31
Zerihun L, Harris M (1983) An electrophysiological analysis of caudally-projecting neurones from the hypothalamic paraventricular nucleus in the rat. Brain Res 261:13–20
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hosoya, Y., Sugiura, Y., Ito, R. et al. Descending projections from the hypothalamic paraventricular nucleus to the A5 area, including the superior salivatory nucleus, in the rat. Exp Brain Res 82, 513–518 (1990). https://doi.org/10.1007/BF00228793
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228793