Abstract
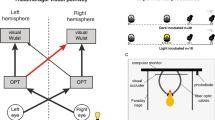
Thalamofugal visual projections of light-incubated chicks are organised asymmetrically. This asymmetry, which is generated by light stimulation of the embryo during the final days of incubation, is sexually dimorphic, being more pronounced in males than in females. We have shown that the development of the asymmetry can be prevented by elevating circulating levels of 17β-oestradiol in the embryo prior to hatching. Light-incubated male chicks were treated 5 days prior to hatching with either one of two doses of 17β-oestradiol (1.5 mg or 2.5 mg in a 0.1 ml solution of 10% ethanol in olive oil) or the vehicle only. After hatching the retrogradely labelling fluorescent dyes True Blue and Fluorogold were injected into the left and right side of the hyperstriatal region of the forebrain, consequently labelling the cell bodies of thalamic neurons which project to this region. Although a pronounced asymmetry was present in the control group, it was not present in both of the 17β-oestradiol-treated groups. These results suggest that the asymmetrical development of thalamofugal visual projections in response to lateralised light stimulation of the embryo is dependent on circulating levels of steroid hormones, and that compared to the male, the lesser degree of asymmetry found in thalamofugal projections of untreated females may be related to the higher levels of circulating oestradiol present in females prior to hatching.
Similar content being viewed by others
References
Adret P, Rogers LJ (1989) Sex difference in the visual projections of young chicks: a quantitative study of the thalamofugal pathway. Brain Res 478:59–73
Andrew RJ, Mench J, Rainey C (1982) Right-left asymmetry in response to visual stimuli in the domestic chick In: Ingle DJ, Goodale MA, Mansfield RJW (eds) Analysis of visual behavior. MIT Press, Cambridge, pp 197–209
Arnold AP (1985) Gonadal steroid-induced organisation and reorganisation of neural circuits involved in bird song. In: Cotman CW (eds) Synaptic plasticity. Guilford Press, New York, pp 263–285
Bottjer SW (1987) Ontogenic changes in the pattern of androgen accumulation in song-control nuclei of male zebra finches. J Neurobiol 18:125–139
Bottjer SW, Dignan TP (1988) Joint hormonal and sensory stimulation modulate neuronal number in adult canary brains. J Neurobiol 19:624–635
Bottjer SW, Schoonmaker JN, Arnold AP (1986) Auditory and hormonal stimulation interact to produce neural growth in adult canaries. J Neurobiol 17:605–612
Boxer MI, Stanford D (1985) Projections to the posterior visual hyperstriatial region in the chick: an HRP study. Exp Brain Res 57:494–498
Ehrlich D, Mark RF (1984) An atlas of the primary visual projections in the brain of the chick Gallus gallus. J Comp Neurol 223:592–610
Gahr M (1990) Delineation of a brain nucleus: comparisons of cytochemical, hodological, and cytoarchitectural views of the song nucleus HVC of the adult canary. J Comp Neurol 294:30–36
Gaston KE, Gaston MG (1984) Unilateral memory after binocular discrimination training: left hemisphere dominance in the chick. Brain Res 303:190–193
Gurusinghe CJ, Ehrlich D (1985) Sex-dependent structural asymmetry of the medial habenular nucleus of the chicken brain. Cell Tissue Res 240:149–152
Karten HJ (1969) The organisation of the avian telencephalon and some speculations on the phylogeny of the amniote telencephalon. Ann NY Acad Sci 167:164–179
Karten HJ, Hodos W, Nauta WJH, Revzin AM (1973) Neural connections of the ‘visual Wulst’ of the avian telencephalon. Experimental syudies in the pigeon Columba livia and owl Speotyto canicularia. J Comp Neurol 150:253–276
Kemali M, Guglielmotti V, Fiorino L (1990) The asymmetry of the habenular nuclei of female and male frogs in spring and in winter. Brain Res 517:251–255
Kolb B, Stewart J (1991) Sex-related differences in dendritic branching of cells in the prefrontal cortex of rats. J Neuroendocrinol 3:96–99
Kuenzel WJ, Mason M (1988) A stereotaxic atlas of the brain of the chicken (Gallus domesticus), Johns Hopkins University Press, Baltimore
Kurz EM, Sengelaub DR, Arnold AP (1986) Androgens regulate dendritic length of mammalian motorneurons in adulthood. Science 232:395–398
Mench JA, Andrew RJ (1986) Lateralisation of a food search task in the domestic chick. Behav Neural Biol 46:107–14
Nordeen EJ, Nordeen kw] (1990) Neurogenesis and sensitive periods in avian song learning. Trends Neurosci 13:31–36
Nordeen EJ, Nordeen kw], Arnold AP (1986) Estrogen establishes sex differences in androgen accumulation in zebra finch brain. J Neurosci 6:734–738
Nordeen EJ, Nordeen kw], Arnold AP (1987) Sexual differentiation of androgen accumulation within the zebra finch brain through selective cell loss and addition. J Comp Neurol 259:393–399
Nottebohm F (1987) Plasticity in adult avian central nervous system: possible relation between hormones, learning and brain repair. In: Mountcastle VB, Plum F, Geiger SR, (eds) Handbook of Physiology, Sec 1, The nervous system, vol V. (American Physiological Society) Oxford University Press, New York, pp 85–108
Nottebohm F (1989) From bird song to neurogenesis. Sci Am, pp 55–61
Rajendra S, Rogers LJ (1992) Asymmetry is present in the thalamofugal visual projections of female chicks. Exp Brain Res, in press
Rogers LJ (1990) Light input and the reversal of functional lateralisation in the chicken brain. Behav Brain Res 38:211–221
Rogers LJ, Bolden SW (1991) Light dependent development and asymmetry of visual projections. Neurosci Lett 121:63–67
Rogers LJ, Sink HS (1988) Transient asymmetry in the projections of the rostral thalamus to the visual hyperstriatum of the chicken, and reversal of its direction by light exposure. Exp Brain Res 70:378–384
Sasaki M, Arnold AP (1991) Androgenic regulation of dendritic trees of motoneurons in the spinal nucleus of the bulbocavernosus: reconstruction after intracellular iontophoresis of horseradish peroxidase. J Comp Neurol 308:11–27
Schwarz IM, Rogers LJ (1992) Testosterone: a role in the development of brain asymmetry in the chick. Neurosci Letts 146:167–170
Tanabe Y, Nakamura T, Fujioka K, Doi O (1979) Production and secretion of sex steroid hormones by the testes, the ovary, and the adrenal glands of the embryonic and young chickens (Gallus domesticus). Gen Comp Endocrinol 39:26–33
Woods JE, Brazzill DM (1981) Plasma 17β-oestradiol levels in the chick embryo. Gen Comp Endocrinol 44:37–43
Woods JE, Simpson RM, Moore PJ (1975) Plasma testosterone levels in the chick embryo. Gen Comp Endocrinol 27:543–547
Zappia JV, Rogers LJ (1987) Sex differences and reversal of brain asymmetry by testosterone in chickens. Behav Brain Res 23:261–267
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rogers, L.J., Rajendra, S. Modulation of the development of light-initiated asymmetry in chick thalamofugal visual projections by oestradiol. Exp Brain Res 93, 89–94 (1993). https://doi.org/10.1007/BF00227783
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227783