Abstract
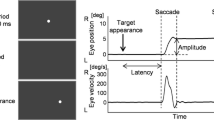
In this study, the execution of delayed saccades in 15 DSM-III-R-schizophrenic patients and 15 normal subjects was investigated. While looking at a central fixation cross, a peripheral target was randomly presented at 10° eccentricity. Subjects were instructed to saccade to the target when the fixation cross was switched off after 500 ms. Two experiments were conducted: (a) a delayed-saccade task and, (b) a memoryguided saccade task, that is, the peripheral target was switched off together with the fixation cross. In the delayed-saccade task, amplitudes of regular saccades did not differ between schizophrenic patients and normals. In the memory-guided saccade task, schizophrenic subjects showed marked hypometric saccades. Incorrect delayed saccades (while the fixation cross was on) were also hypometric in schizophrenics, but not in normal controls. The final eye position, i.e., the position reached after the execution of correction saccades, however, did not differ between patients and controls. This means that schizophrenics show a deficit in the programming of primary saccades, if the fixation point and the peripheral target are (a) both visually presented or (b) both memorized. The results support the hypothesis that these saccades are the result of an averaging effect between the fixation point and the peripheral target. It is further hypothesized that these deficits might be explained by a lack of prefrontal inhibition of ocular fixation areas.
Similar content being viewed by others
References
American Psychiatric Association (1987) Diagnostic and statistical manual of mental disorders, 3rd edn, revised (DSM-III-R). American Psychiatric Association, Washington, DC
Coren S, Hoenig P (1972) Effect of non-target stimuli upon length of voluntary saccades. Percept Mot Skills 34: 499–508
Crawford TJ, Henderson L, Kennard C (1993) Abnormalities of nonvisually guided eye movements in Parkinson's disease. Brain 112: 1573–1586
Diefendorf AR, Dodge R (1908) An experimental study of the ocular reactions of the insane from photographic records. Brain 31: 451–489
Dodge R (1902) Five types of eye movement in the horizontal plane of the field of regard. Am J Physiol 8: 307–329
Done DJ, Frith CD (1989) Automatic and strategic volitional saccadic eye movements in psychotic patients. Eur Arch Psychiatry Neurol Sci 239: 27–32
Everling S, Ott D, Holtermann P, Flohr H (1992) Scanning laser ophthalmoscopy: a new tool for studying eye movements via direct retinal stimulation and fundus monitoring. In: Elsner N, Richter DW (eds) Rhythmogenesis in neurones and networks. Thieme, Stuttgart, p 746
Findlay JM (1982) Global visual processing for saccadic eye movements. Vision Res 22: 1033–1045
Fischer B, Weber H (1993) Express saccades and visual attention. Behav Brain Sci 16: 553–610
Fukushima J, Fukushima K, Chiba T, Tanaka S, Yamashita I, Kato M (1988) Disturbances of voluntary control of saccadic eye movements in schizophrenic patients. Biol Psychiatry 23: 670–677
Fukushima J, Fukushima K, Morita N, Yamashita I (1990) Further analysis of the control of voluntary saccadic eye movements in schizophrenic patients. Biol Psychiatry 28: 943–958
Funahashi S, Bruce CJ, Goldman-Rakic PS (1989) Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349
Fuster JM (1991) The prefrontal cortex: anatomy, physiology, and neurophysiology of the frontal lobe. Raven, New York
Glimcher PW, Sparks DL (1993) Representation of averaging saccades in the superior colliculus of the monkey. Exp Brain Res 95: 429–435
Goldberg ME, Bushnell MC, Bruce CJ (1986) The effect of attentive fixation on eye movements evoked by electrical stimulation of the frontal eye fields. Exp Brain Res 61: 579–584
Goldman PS, Nauta WJH (1976) Autoradiographic demonstration of a projection from prefrontal association cortex to the superior colliculus in the rhesus monkey. Brain Res 116: 145–149
Goldman PS, Nauta WJH (1977) An intricately patterned prefronto-caudate projection in the rhesus monkey. J Comp Neurol 171: 369–385
Goldman-Rakic PS (1991) Prefrontal cortical dysfunction in schizophrenia: the relevance of working memory. In: Carroll BJ, Barrett JE (eds) Psychopathology and the brain. Raven, New York, pp 1–23
Guitton D, Buchtel HA, Douglas RM (1985) Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res 58: 455–472
Guitton D (1991) Control of saccadic eye movements by the superior colliculus and basal ganglia. In: Carpenter RHS (ed) Eye movements. Macmillian, London, pp 244–276
Hallett PE (1978) Primary and secondardy saccades to goals defined by instructions. Vision Res 18: 1279–1296
Hallett PE, Adams BD (1980) The predictability of saccadic latency in a novel voluntary oculomotor task. Vision Res 20: 329–339
Holzman PS, Proctor LR, Hughes DW (1973) Eye tracking patterns in schizophrenia. Science 181: 179–181
Holzman PS, Proctor LR, Levy DE, Yasillo JJ, Meltzer HY, Hurt SW (1974) Eye tracking dysfunctions in schizophrenic patients and their relatives. Arch Gen Psychiatry 31: 143–151
Ingvar, DH, Franzen G (1974) Abnormalities of cerebral blood flow distribution in patients with chronic schizophrenia. Acta Psychiatr Scand 50: 425–462
Kapoula Z (1985) Evidence for a range effect in the saccadic system. Vision Res 25: 1155–1157
Leichnetz GR, Spence RF, Hardy SGP, Astruc J (1981) The prefrontal corticotectal projection in the monkey; an anterograde and retrograde horseradish peroxidase study. Neuroscience 6: 1023–1041
Levin S (1984) Frontal lobe dysfunctions in schizophrenia. I. Eye movement impairments. J Psychiatr Res 18: 27–55
Levy DL, Holzman PS, Matthysse S, Mendell NR (1993) Eye tracking dysfunction and schizophrenia: a critical perspective. Schizophr Bull 19: 461–536
Mackert A, Flechtner M (1989) Saccadic reaction times in acute and remitted schizophrenics. Eur Arch Psychiatry Neurol Sci 239: 33–38
Mather JA, Putchat C (1983) Motor control of schizophrenics. I. Oculomotor control of schizophrenics: a deficit in sensory processing-not strictly in motor control. J Psychiatr Res 17: 343–360
Munoz DP, Wurtz RH (1993a) Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol 70: 559–575
Munoz DP, Wurtz RH (1993b) Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J Neurophysiol 70: 576–589
Paré M, Crommelinck M, Guitton D (1994) Gaze shifts evoked by stimulation of the superior colliculus in the head-free cat conform to the motor map but also depend on stimulus strength and fixation activity. Exp Brain Res 101: 123–139
Park S, Holzman PS (1992) Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry 49: 975–982
Robinson DA (1972) Eye movements evoked by collicular stimulation in the alert monkey. Vision Res 12: 1795–1808
Robinson DA, Fuchs AF (1968) Eye movements evoked by stimulation of frontal eye fields. J Neurophysiol 32: 637–648
Rosse RB, Schwartz BL, Kim SY, Deutsch SI (1993) Correlation between antisaccade and Wisconsin Card Sorting Test performance in schizophrenia. Am J Psychiatry 150: 333–335
Sawaguchi T, Goldman-Rakic PS (1991) D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 251:947–950
Schiller PH, Sandell JH (1983) Interactions between visually and electrically elicited saccades before and after superior colliculus and frontal eye field ablations in the rhesus monkey. Exp Brain Res 49: 381–392
Schmid-Burgk, W, Becker W, Diekmann V, Jürgens R, Kornhuber HH (1982) Disturbed smooth pursuit and saccadic eye movements in schizophrenia. Arch Psychiatr Nervenkr 232: 381–389
Sparks DL, Mays LE (1983) Spatial localization of saccade targets. I. Compensation for stimulation-induced perturbations in eye position. J Neurophysiol 49: 45–63
Thaker GK, Nguyen JA, Tamminga CA (1989) Saccadic distractibility in schizophrenic patients with tardive dyskinesia. Arch Gen Psychiatry 46: 755–756
Weber H, Latanov A, Fischer B (1993) Context dependent amplitude modulations of express and regular saccades in man and monkey. Exp Brain Res 93: 335–344
Weinberger DR, Berman KF, Zec RF (1986) Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. Arch Gen Psychiatry 43: 114–124
Weinberger DR, Berman KF, Daniel DG (1991) Prefrontal cortex dysfunction in schizophrenia. In: Levin HS, Eisenberg HM, Benton AL (eds) Frontal lobe function and dysfunction. Oxford University Press, New York, pp 275–287
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Everling, S., Krappmann, P., Preuss, S. et al. Hypometric primary saccades of schizophrenics in a delayed-response task. Exp Brain Res 111, 289–295 (1996). https://doi.org/10.1007/BF00227306
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227306