Abstract
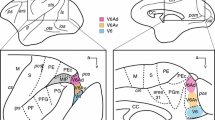
The cortical and thalamic afferent connections of rat orbital cortex were investigated using fluorescent retrograde axonal tracers. Each of the four orbital areas has a distinct pattern of connections. Corticocortical connections involving the ventral and ventrolateral orbital areas are more extensive than those of the medial and lateral orbital areas. The medial orbital area has cortical connections with the cingulate, medial agranular (Fr2) and posterior parietal (PPC) cortices. The ventral orbital area has connections with the cingulate area, area Fr2, secondary somatic sensory area Par2, PPC, and visual areas Oc2M and Oc2L. The ventrolateral orbital area (VLO) receives cortical input from insular cortex, area Fr2, somatic sensory areas Par1 and Par2, PPC and Oc2L. The lateral orbital area has cortical connections limited to the agranular and granular insular areas, and Par2. Thalamic afferents to the four orbital fields are also topographically organized, and are focused in the submedial and mediodorsal nuclei. The ventrolateral orbital area receives input from the entirety of the submedial nucleus, whereas the other orbital areas receive input from its periphery only. Each orbital area is connected with a particular segment of the mediodorsal nucleus. The medial orbital area receives its principal thalamic afferents from the parataenial nucleus, the dorsocentral portion of the mediodorsal nucleus, and the ventromedial portion of the submedial nucleus. The ventral orbital area receives input from the lateral segment of the mediodorsal nucleus, the rostromedial portion of the submedial nucleus and the central lateral nucleus. Thalamic afferents to the ventrolateral orbital area arise from the entirety of the submedial nucleus and from the lateral segment of the mediodorsal nucleus. The lateral orbital area receives thalamic afferents from the central segment of the mediodorsal nucleus, the ventral portion of the submedial nucleus and the ventromedial nucleus. The paraventricular, ventromedial, rhomboid and reuniens nuclei also provide additional input to the four orbital areas. The connections of the ventrolateral orbital area are interpreted in the context of its role in directed attention and allocentric spatial localization. The present findings provide anatomical support for the view that areas Fr2, PPC and VLO comprise a cortical network mediating such functions.
Similar content being viewed by others
References
Backonja M, Miletic V (1991) Responses of neurons in the rat ventrolateral orbital cortex to phasic and tonic nociceptive stimulation. Brain Res 557: 353–355
Beckstead RM (1979) An autoradiographic examination of corticocortical and subcortical projections of the mediodorsal-projection (prefrontal) cortex in the rat. J Comp Neurol 184: 43–62
Berendse HW, Groenewegen HJ (1991) Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience 42: 73–102
Chandler HC, King V, Corwin JV, Reep RL (1992) Thalamocortical connections of rat posterior parietal cortex. Neurosci Lett 143: 237–242
Coffield JA, Bowen KK, Miletic V (1992) Retrograde tracing of projections between the nucleus submedius, the ventrolateral orbital cortex, and the midbrain in the rat. J Comp Neurol 321: 488–499
Corwin JV, Kanter S, Watson RT, Heilman KM, Valenstein E, Hashimoto A (1986) Apomorphine has a therapeutic effect on neglect produced by unilateral dorsomedial prefrontal cortex lesions in rats. Exp Neurol 94: 683–698
Corwin JV, Fussinger M, Meyer RC, King V, Reep RL (1994) Bilateral destruction of the ventrolateral orbital cortex produces allocentric but not egocentric spatial deficits. Behav Brain Res 61: 79–86
Crowne DP, Pathria MN (1982) Some attentional effects of unilateral frontal lesions in the rat. Behav Brain Res 6: 25–39
Crowne DP, Richardson CM, Dawson KA (1986) Parietal and frontal eye field neglect in the rat. Behav Brain Res 22: 227–231
Crowne DP, Novotny MF, Maier SE, Vitols SR (1992) Effects of unilateral parietal lesions on spatial localization in the rat. Behav Neurosci 106: 811–822
Dostrovsky JO, Guilbaud G (1988) Noxious stimuli excite neurons in nucleus submedius of the normal and arthritic rat. Brain Res 460: 269–280
Follett KA, Dirks B (1995) Responses of neurons in ventrolateral orbital cortex to noxious visceral stimulation in the rat. Brain Res 669: 157–162
Gerfen CR, Clavier RM (1979) Neural inputs to the prefrontal agranular insular cortex in the rat: horseradish peroxidase study. Brain Res Bull 4: 347–353
Groenewegen HJ (1988) Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 24: 379–431
Herkenham M (1978) The connections of the nucleus reuniens thalami: evidence for a direct thalamo-hippocampal pathway in the rat. J Comp Neurol 177: 589–610
Herkenham M (1979) The afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J Comp Neurol 183: 487–518
Hutchison WD, Harfa A, Dostrovsky JO (1995) Ventrolateral orbital cortex and periaqueductal gray stimulation-induced effects on on- and off-cells in the rostral ventromedial medulla in the rat. Neuroscience 70: 391–407
Jones EG, Leavitt RY (1974) Retrograde axonal transport and the demonstration of non-specific projections to the cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat and monkey. J Comp Neurol 154: 349–378
Kesner RP, Farnsworth G, DiMattia BV (1989) Double dissociation of egocentric and allocentric space following prefrontal and parietal cortex lesions in the rat. Behav Neurosci 103: 956–961
King V, Corwin JV (1992) Spatial deficits and hemispheric asymmetries in the rat following unilateral and bilateral lesions of posterior parietal or medial agranular cortex. Behav Brain Res 143:237–242
King V, Corwin JV (1993) Comparison of hemiinattention produced by unilateral lesions of posterior parietal cortex or medial agranular prefrontal cortex in rats: neglect, extinction, and the role of stimulus distance. Behav Brain Res 54: 117–131
King V, Corwin JV, Reep RL (1989) Production and characterization of neglect in rats with unilateral lesions of ventrolateral orbital cortex. Exp Neurol 105: 287–299
Kolb B (1984) Functions of the frontal cortex of the rat: a comparative review. Brain Res Rev 8: 65–98
Kolb B (1990) Prefrontal cortex. In: Kolb B, Tees RC (eds) The cerebral cortex of the rat. MIT, Cambridge, Mass., pp 437–458
Kolb B, Nonneman AJ (1974) Frontolimbic lesions and social behavior in the rat. Physiol Behav 13: 637–643
Kolb B, Whishaw IQ, Schallert T (1977) Aphagia, behavior sequencing, and body weight set point following orbital frontal lesions in rats. Physiol Behav 19: 93–103
Kolb B, Sutherland RJ, Whishaw IQ (1983) A comparison of the contributions of the frontal and parietal association cortex to spatial localization in rats. Behav Neurosci 97: 13–27
Krettek JE, Price JL (1977) The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171: 157–192
Miletic V, Coffield JA (1989) Responses of neurons in the rat nucleus submedius to noxious and innocuous mechanical cutaneous stimuli. Somatosens Mot Res 6: 567–587
Miller MW, Vogt BA (1984) Direct connections of rat visual cortex with sensory, motor, and association cortices. J Comp Neurol 226: 184–202
Nonneman AJ, Corwin JV (1981) Differential effects of prefrontal cortex ablation in neonatal, juvenile, and young adult rats. J Comp Physiol Psychol 23: 588–602
Paperna T, Malach R (1991) Patterns of sensory intermodality relationships in the cerebral cortex of the rat. J Comp Neurol 308:432–456
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, Sydney
Price JL, Slotnick BM (1983) Dual olfactory representation in the rat thalamus: an anatomical and electrophysiological study. J Comp Neurol 215: 63–77
Ray JP, Price JE (1992) The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. J Comp Neurol 323: 167–197
Reep RE, Winans SS (1982a) Afferent connections of dorsal and ventral agranular insular cortex in the hamster Mesocricetus auratus. Neuroscience 7: 1265–1288
Reep RE, Winans SS (1982b) Efferent connections of dorsal and ventral agranular insular cortex in the hamster, Mesocricetus auratus. Neuroscience 7: 2609–2635
Reep RE, Corwin JV, Hashimoto A, Watson RT (1984) Afferent connections of medial precentral cortex in the rat. Neurosci Eett 44: 247–252
Reep RE, Corwin JV, Hashimoto A, Watson RT (1987) Efferent connections of the rostral portion of medial agranular cortex in rats. Brain Res Bull 19: 203–221
Reep RE, Goodwin GS, Corwin JV (1990) Topographic organization in the corticocortical connections of medial agranular cortex in rats. J Comp Neurol 294: 262–280
Reep RE, Chandler HC, King V, Corwin JV (1994) Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp Brain Res 100: 67–84
Sanderson KJ, Dreher B, Gayer N (1991) Prosencephalic connections of striate and extrastriate areas of rat visual cortex. Exp Brain Res 85: 324–334
Saper CB (1982) Convergence of autonomie and limbic connections in the insular cortex of the rat. J Comp Neurol 210: 163–173
Selemon ED, Goldman-Rakic PS (1988) Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci 8: 4049–4068
Van Eden CG, Lamme VAF, Uylings HBM (1992) Heterotopic cortical afferents to the medial prefrontal cortex in the rat. A combined retrograde and anterograde tracing study. Eur J Neurosci 4: 77–97
Vargo JM, Corwin JV, King V, Reep RE (1988) Hemispheric asymmetry in neglect produced by unilateral lesions of dorsomedial prefrontal cortex in rats. Behav Neurosci 102: 199–209
Vargo JM, Richard-Smith M, Corwin JV (1989) Spiroperidol reinstates asymmetries in neglect in rats recovered from left vs. right dorsomedial prefrontal cortex lesions. Behav Neurosci 103: 1786–1796
Vogt BA, Miller MW (1983) Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol 216: 192–210
Wilcott RC (1981) Medial and orbital cortex and the suppression of behavior in the rat. Physiol Behav 27: 237–241
Yoshida A, Dostrovsky JO, Sessle BJ, Chiang CY (1991) Trigeminal projections to the nucleus submedius of the thalamus in the rat. J Comp Neurol 307: 609–625
Yoshida A, Dostrovsky JO, Chiang CY (1992) The afferent and efferent connections of the nucleus submedius in the rat. J Comp Neurol 324: 115–133
Zilles K (1985) The cortex of the rat: a stereotaxic atlas. Springer, Berlin Heidelberg New York
Zilles K, Wree A (1985) Cortex: areal and laminar structure. In: Paxinos G (ed) The rat nervous system: forebrain and midbrain, vol 1. Academic Press, Sydney, pp 375–415
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reep, R.L., Corwin, J.V. & King, V. Neuronal connections of orbital cortex in rats: topography of cortical and thalamic afferents. Exp Brain Res 111, 215–232 (1996). https://doi.org/10.1007/BF00227299
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227299