Abstract
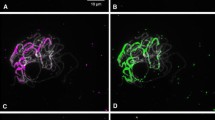
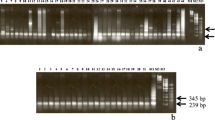
A species-specific repetitive DNA fragment has been isolated from a genomic library of Solanum brevidens. Sequence analysis revealed a regular organization of three non-homologous subrepeats forming tandemly-arranged composite repetitive units. Interpretation of Southern hybridization patterns based on the known sequence data suggests that the isolated sequence element represents an abundant organization type, although the presence of simple tandem arrays of the subrepeats is also indicated. Seventy-four percent sequence similarity was found between one of the S. brevidens subrepeats (Sb4AX) and a satellite DNA (TGRI) localized as a subtelomeric repeat on almost all Lycopersicon esculentum chromosomes. Insitu hybridization indicated that, similarly to TGRI, the S. brevidens-specific repeats are located at the ends of the arms of several chromosomes. On the basis of the data obtained, a common ancestral sequence can be proposed for the tomato (TGRI) and the S. brevidens (Sb4AX) repeat however, the molecular organization of this element in these two species evolved in a basically different manner.
Similar content being viewed by others
References
Appels R, Driscoll C, Peacock WJ (1978) Heterochromatin and highly-repeated DNA sequences in rye (Secale cereale). Chromosoma 70:67–89
Arnheim N (1983) Concerted evolution of multigene families. In: Koehn R, Nei M (eds) Evolution of genes and proteins. Sinauer, Sunderland, pp 38–61
Bedbrook JR, Jones J, O'Dell M, Thompson RD, Flavell RB (1980) A molecular description of telomeric heterochromatin in Secale species. Cell 19:545–560
Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890
Dhar MS, Dabak MM, Gupta VS, Ranjekar PK (1988) Organisation and properties of repeated DNA sequences in the rice genome. Plant Sci 55:43–52
Doolittle WF, Sapienza C (1980) Selfish genes, the phenotype paradigm and genome evolution. Nature 284:111–117
Dvorak J, McGuire PE, Cassidy B (1988) Apparent sources of the A genomes of wheats inferred from polymorphism in abundance and restriction fragment length of repeated nucleotide sequences. Genome 30:680–689
Evans IJ, James AM, Barnes SR (1983) Organization and evolution of repeated DNA sequences in closely-related plant genomes. J Mol Biol 170:803–826
Feinberg AP, Vogenstein B (1983) A technique for readiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132:6–13
Flavell R (1980) The molecular characterization and organization of plant chromosomal DNA sequences. Annu Rev Plant Physiol 31:569–596
Flavell R (1986) Repetitive DNA and chromosome evolution in plants. Phil Trans R Soc Lond B 312:227–242
Ganal MW, Lapitan NLV, Tanksley SD (1988) A molecular and cytogenetic survey of major repeated DNA sequences in tomato (Lycopersicon esculentum). Mol Gen Genet 213:262–268
Ganal MW, Lapitan NLV, Tanksley SD (1991) Macrostructure of the tomato telomeres. Plant Cell 3:87–94
Grellet F, Delcasso D, Panabieres H, Delseny M (1986) Organization and evolution of a higher plant alphoid-like satellite DNA sequence. J Mol Biol 187:495–507
Gupta PK, Fedak G, Molnar SJ, Wheatcroft R (1989) Distribution of a Secale cereale DNA repeat sequence among 25 Hordeum species. Genome 32:383–388
Hawkes JG (1990) The potato: evolution, biodiversity and genetic resources. Belhaven Press, London, and Smithsonian Institution Press, Washington, D.C.
Hermsen JGT, Taylor LM (1979) Successful hybridization of nontuberous Solanum etuberosum Lind. and tuber-bearing S. pinnatisectum Dun. Euphytica 28:1–7
Hosaka K, Ogihara Y, Matsubayashi M, Tsunewaki K (1984) Phylogenetic relationship between the tuberous Solanum species as revealed by restriction endonuclease analysis of chloroplast DNA. Jpn J Genet 59:349–369
Hutchinson J, Lonsdale DM (1982) The chromosomal distribution of cloned highly-repetititive sequences from hexaploid wheat. Heredity 48:371–376
Lapitan NLV (1992) Organization and evolution of higher plant nuclear genomes. Genome 35:171–181
Martinez-Zapater J, Estelle M, Sommerville C (1986) A highly-repeated DNA sequence in Arabidopsis thaliana. Mol Gen Genet 204:417–423
McIntyre CI, Clarke BC, Appels R (1988) Amplification and dispersion of repeated DNA sequences. Plant Syst Evol 160:39–60
Orgel LE, Crick FHC (1980) Selfish DNA: the ultimate parasite. Nature 284:604–607
Peacock WJ, Dennis ES, Rhoades MM, Pryor AJ (1981) Highly-repeated DNA sequence limited to knob heterochromatin in maize. Proc Natl Acad Sci USA 78:4490–4494
Pehu E, Thomas M, Poutala T, Karp A, Jones MGK (1990) Speciesspecific sequences in the genus Solanum: identification, characterization, and application to study somatic hybrids of S. brevidens and S. tuberosum. Theor Appl Genet 80:693–698
Pinkel D, Straume T, Gray JW (1986) Cytogenetic analysis using quantitative, high-sensitivity fluorescence hybridization. Proc Natl Acad Sci USA 83:2934–2938
Preiszner J, Fehér A, Veisz O, Sutka J, Dudits D (1991) Characterization of morphological variation and cold resistance in interspecific somatic hybrids between potato (Solanum tuberosum) and S. brevidens. Euphytica 57:37–49
Ramanna MS (1988) Strategies for cloning useful genes from Solanaceous crops. In: Louwes KM, Toussaint HAJM, Dellaert LMW (eds), Parental line breeding and selection in potato breeding. Pudoc, Wageningen, pp 118–122
Saiga H, Edström JE (1985) Long tandem arrays of complex repeat units in Chironomus telomeres. EMBO J 4:799–804
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schmidt T, Jung C, Metzlaff M (1991) Distribution and evolution of two satellite DNAs in the genus Beta. Theor Appl Genet 82:793–799
Schweizer G, Ganal M, Ninnemann H, Hemleben V (1988) Speciesspecific DNA sequences for identification of somatic hybrids between Lycopersicon esculentum and Solanum acaule. Theor Appl Genet 75:679–684
Sibson DR, Hughes SG, Bryant JA, Fitchett PN (1991) Sequence organization of simple, highly-repetitive DNA elements in Brassica species. J Exp Bot 42:243–249
Singer MF (1982) Highly-repeated sequences in mammalian genomes. Int Rev Cyt 76:67–112
Solano R, Hueros G, Fominaya A, Ferrer E (1992) Organization of repeated sequences in species of the genus Avena. Theor Appl Genet 83:602–607
Spooner DM, Anderson GJ, Jansen RK (1993) Chloroplast DNA evidence for the interrelationships of tomatoes, potatoes, and pepinos (Solanaceae). Am J Bot 80:676–688
Vershinin AV, Salina EA, Solovyov VV, Timofeyeva LL (1990) Genomic organization, evolution and peculiarities of highly-repetitive DNA of Hordeum vulgare. Genome 33:441–449
Vincze É, Kiss GB (1990) A phosphate group at the cos ends of phage lambda DNA is not a prerequisite for in-vitro packaging: an alternative method for constructing genomic libraries using a new phasmid vector, lpGY97. Gene 96:17–22
Wu HK, Chung MC, Wu T, Ning CN, Wu R (1991) Localization of specific repetitive DNA sequences in individual rice chromosomes. Chromosoma 100:330–338
Zamir D, Tanksley SD (1988) The tomato genome is comprised largely of fast-evolving, low-copy-number sequences. Mol Gen Genet 213:254–261
Author information
Authors and Affiliations
Additional information
Communicated by Y. Gleba
Rights and permissions
About this article
Cite this article
Preiszner, J., Takács, I., Bilgin, M. et al. Organization of a Solatium brevidens repetitive sequence related to the TGRI subtelomeric repeats of Lycopersicon esculentum . Theoret. Appl. Genetics 89, 1–8 (1994). https://doi.org/10.1007/BF00226974
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00226974