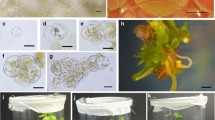
Summary
Protoplast fusion was used to combine the cytoplasmic traits of atrazine resistance and male sterility in Brassica oleracea var. italica (broccoli). Leaf protoplasts from broccoli with the petaloid B. nigra type of cytoplasmic male sterility were fused with hypocotyl protoplasts from an atrazine-resistant biotype of B. campestris var. oleifera cv Candle (oilseed rape). A total of 19 colonies regenerated shoots, all of which were broccolilike in phenotype, i.e., lacked trichomes. Four shoots, all from one colony, were atrazine resistant, surviving and growing in the presence of 25 μM atrazine. A leaf piece assay also confirmed that they were atrazine resistant. Molecular analysis showed that they contain chloroplasts from the atrazine-resistant B. campestris parent and mitochondria from the B. nigra parent. No recombination or rearrangement of the mitochondrial genomes in the fusion products was detected. These four plants and their progeny all showed the petaloid B. nigra type of male sterility.
Similar content being viewed by others
References
Ahrens WH, Stoller EW (1983) Competition, growth rate and CO2 fixation in triazine-susceptible and -resistant smooth pigweed (Amaranthus hybridus). Weed Sci 31:438–444
Bannerot H, Boulidard L, Cauderon V, Tempe J (1974) Transfer of cytoplasmic male sterility from Raphanus sativus to Brassica oleracea. In: Proc. Eucarpia Meeting-Cruciferae, Scottish Hortic Res. Inst. Invergavrie, A. B. Wills and C. North, UK, pp 52–54
Bannerot H, Boulidard L, Chupeau Y (1977) Unexpected difficulties met with the radish cytoplasm in Brassica oleracea. Cruciferae Newslett 2:16
Barsby TL, Chuong PV, Yarrow SA, Wu S-C, Coumans M, Kemble RJ, Powell AD; Beversdorf WD, Pauls KP (1987 a) The combination of Polima cms and cytoplasmic triazine resistance in Brassica napus. Theor Appl Genet 73:809–814
Barsby TL, Kemble RJ, Yarrow SA (1987b) Brassica cybrids and their utility in plant breeding. In: Wettstein D von, Chua N-H (eds) Plant molecular biology. Plenum Press, New York, pp 223–234
Beversdorf WD, Weiss-Lerman J, Erickson LR, Souza Machado V (1980) Transfer of cytoplasmically-inherited triazine resistance from Bird's rape to cultivated oilseed rape (Brassica campestris and B. napus). Can J Genet Cytol 22:167–172
Beversdorf WD, Erickson LR, Grant I (1985) Hybridization process utilizing a combination of cytoplasmic male sterility and herbicide tolerance. US Patent 4,517,763
Chatterjee G, Sikdar SR, Das S, Sen SK (1988) Intergeneric somatic hybrid production through protoplast fusion between Brassica juncea and Diplotaxis muralis. Theor Appl Genet 76:915–922
Christey MC (1989) Cell and tissue culture studies of Brassica oleracea and B. campestris. PhD thesis, Cornell University, Ithaca, NY
Chuong PV, Beversdorf WD, Powell AD, Pauls KP (1988) Somatic transfer of cytoplasmic traits in B. napus L. by haploid protoplast fusion. Mol Gen Genet 211:197–201
Conard SG, Radosevich SR (1979) Ecological fitness of Senecio vulgaris and Amaranthus retroflexus biotypes susceptible or resistant to atrazine. J Appl Ecol 16:171–177
Cseplo A, Medgyesy P, Hideg E, Demeter S, Marton L, Maliga P (1985) Triazine-resistant Nicotiana mutants from photomixotrophic cell cultures. Mol Gen Genet 200:508–510
Dellaporta SL, Wood J, Hicks JB (1985) Maize DNA miniprep. In: Malmberg R, Messing J, Sussex I (eds) Molecular biology of plants. Cold Spring Harbor Laboratory Press, Cold Spring Harbor/NY, pp 36–37
Dickson MH (1975) G1117A, G1102A, and G1106A cytosterile broccoli inbreds. Hortic Sci 10:535
Dickson MH, Kyle M (1987) Seed production on cytosterile Brassica oleracea plants with B. nigra cytoplasm. Cruciferae Newslett 12:45
Galun E, Aviv D, Breiman A, Fromm H, Perl A, Vardi A (1987) Cybrids in Nicotiana, Solanum and Citrus: isolation and characterization of plastome mutants: pre-fusion treatments, selection and analysis of cybrids. In: Wettstein D von, Chua N-H (eds) Plant moleclar biology. Plenum Press, New York, pp 199–222
Glimelius K, Djupsjobacka M, Fellner-Feldegg H (1986) Selection and enrichment of plant protoplast heterokaryons of Brassicaceae by flow sorting. Plant Sci 45:133–141
Grant I, Beversdorf WD (1985) Agronomic performance of triazine-resistant single-cross hybrid oilseed rape (Brassica napus L.). Can J Plant Sci 65:889–892
Gressel J, Ben-Sinai G (1985) Low intraspecific competitive fitness in a triazine-resistant, nearly nuclear-isogenic line of Brassica napus. Plant Sci 38:29–32
Hoser-Krauze J, Antosik J (1987) Horticultural value and seed setting of cytoplasmic male-sterile cauliflower lines with Raphanus sativus CMS (Bannerot). Cruciferae Newslett 12:34–35
Jarl CI, Bornman CH (1988) Correction of chlorophyll-defective, male-sterile winter oilseed rape (Brassica napus) through organelle exchange: phenotypic evaluation of progeny. Hereditas 108:97–102
Jourdan PS, Earle ED, Mutschler MA (1989 a) Atrazine-resistant cauliflower obtained by somatic hybridization between Brassica oleracea and B. napus. Theor Appl Genet 78:271–279
Jourdan PS, Earle ED, Mutschler M (1989 b) Synthesis of male sterile, triazine-resistant Brassica napus by somatic hybridization between cytoplasmic male sterile B. oleracea and atrazine-resistant B. campestris. Theor Appl Genet 78:445–455
Kolodner R, Tewari KK (1972) Physiochemical characterization of mitochondrial DNA from pea leaves. Proc Natl Acad Sci USA 69:1830–1834
Linsmaier EM, Skoog F (1965) Organic growth factor requirments of tobacco tissue cultures. Physiol Plant 18:100–127
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor/NY
Mapplebeck LR, Souza-Machado V, Gronzinski B (1982) Seed germination and seedling growth characteristics of atrazinesusceptible and -resistant biotypes of Brassica campestris. Can J Plant Sci 62:733–739
Menczel L, Morgan A, Brown S, Maliga P (1987) Fusion-mediated combination of Ogura-type cytoplasmic male sterility with Brassica napus plastids using X-irradiated CMS protoplasts. Plant Cell Rep 6:98–101
Morgan A, Maliga P (1987) Rapid chloroplast segregation and recombination of mitochondrial DNA in Brassica cybrids. Mol Gen Genet 209:240–246
Palmer JD, Herbon LA (1988) Plant mitochondrial DNA evolves rapidly in structure, but slowly in sequence. J Mol Evol 28:87–97
Pearson OH (1972) Cytoplasmically inherited male sterility characters and flavor components from the species cross Brassica nigra (L.) Koch × B. oleracea L. Am Soc Hortic Sci 97:397–402
Pelletier G (1989) Organelle manipulation by cybridization: methods, results and applications. In: Quiros CF, McGuire PE (eds) Proc 5th Crucifer Genet Workshop, University of California, Davis, April 7–9, pp 15–16
Pelletier G, Primard C, Vedel F, Chetrit P, Remy R, Rousselle P, Renard M (1983) Intergeneric cytoplasmic hybridization in Cruciferae by protoplast fusion. Mol Gen Genet 191:244–250
Radosevich SR, Holt JS (1982) Physiological responses and fitness of susceptible and resistant weed biotypes to triazine herbicides. In: LeBaron HM, Gressel J (eds) Herbicide resistance in plants. Wiley and Sons, New York, pp 163–183
Röbbelen G (1987) Measurement of yield penalty for triazine tolerance in oilseed rape, B. Napus L. Cruciferae Newslett 12:124–125
Robertson D, Earle ED (1986) Plant regeneration from leaf protoplasts of B. oleracea var. italica cv Green Comet broccoli. Plant Cell Rep 5:61–64
Robertson D, Earle ED (1987) Nitro-blue tetrazolium: a specific stain for photosynthetic activity in protoplasts. Plant Cell Rep 6:70–73
Schenck HR, Röbbelen G (1982) Somatic hybrids by fusion of protoplasts from Brassica oleracea and B. campestris. Z. Pflanzenzüchtung 89:278–288
Schonfeld M, Yaacoby T, Michael O, Rubin B (1987) Triazine resistance without reduced vigor in Phalaris paradoxa. Plant Physiol 83:329–333
Walters TW, Earle ED, Dickson MH (1990) Male-sterile, coldtolerant cauliflower. In: McFerson JR, Kresovich S, Dwyer SG (eds) Proc. 6th Crucifer Genet Workshop, Cornell University, Ithaca NY October 6–9, p 41
Wendel JF, Weeden NF (1989) Visualization and interpretation of plant isozymes. In: Soltis D, Soltis P (eds) Isozymes in plant biology. Dioscaorides Press, Washington/D.C.
Yarrow SA, Wu SC, Barsby TL, Kemble RJ, Shepard JF (1986). The introduction of CMS mitochondria to triazine tolerant Brassica napus L. var. “Regent”, by micromanipulation of individual heterokaryons. Plant Cell Rep 5:415–418
Yarrow SA, Burnett LA, Wildemann RP, Kemble RJ (1990) The transfer of ‘Polima’ cytoplasmic male sterility from oilseed rape (Brassica napus) to broccoli (B. oleracea) by protoplast fusion. Plant Cell Rep 9:185–188
Young EG, Hanson MR (1987) A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell 50:41–49
Author information
Authors and Affiliations
Additional information
Communicated by P. Maliga
Rights and permissions
About this article
Cite this article
Christey, M.C., Makaroff, C.A. & Earle, E.D. Atrazine-resistant cytoplasmic male-sterile-nigra broccoli obtained by protoplast fusion between cytoplasmic male-sterile Brassica oleracea and atrazine-resistant Brassica campestris . Theoret. Appl. Genetics 83, 201–208 (1991). https://doi.org/10.1007/BF00226252
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00226252