Summary
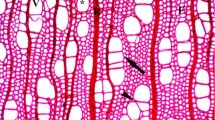
Regions of spiral vascular tissues and circular vessels occur normally in branch junctions. Their size and frequency increase continuously with age and stem width. This phenomenon is general and was found in all the 15 species studied. The differentiation of narrow spiral vessels with non-functional circular vessels decreases water conductivity through branch junctions leading to hydraulic segmentation of lateral branches from the main stem. Possible hormonal mechanisms controlling circular vascular patterns and narrow vascular elements are discussed.
Similar content being viewed by others
References
Aloni R (1987a) The induction of vascular tissues by auxin. In: Davis PJ (ed) Plant hormones and their role in plant growth and development. Nijhoff, Dordrecht, pp 363–374
Aloni R (1987b) Differentiation of vascular tissues. Annu Rev Plant Physiol 38: 179–204
Aloni R (1990) Wood formation in deciduous hardwood trees. In: Raghavendra AS (ed) Physiology of trees. Wiley, New York (in press)
Aloni R, Wolf A (1984) Suppressed buds embedded in the bark across the bole and the occurrence of their circular vessels in Ficus religiosa. Am J Bot 71: 1060–1066
Aloni R, Zimmermann MH (1983) The control of vessel size and density along the plant axis — a new hypothesis. Differentiation 24: 203–208
Aloni R, Zimmermann MH (1984) Length, width and pattern of regenerative vessels along strips of vascular tissues. Bot Gaz 154: 50–54
Barrows-Broaddus J, Dwinell LD (1983) Histopathology of Fusarium moniliforme var. subglutinans in four species of southern pines. Phytopathology 73: 882–889
Chattaway MM (1958) Bud development and lignotuber formation in Eucalypts. Aust J Bot 6: 103–115
Eames AJ, MacDaniels LH (1947) An introduction to plant anatomy. 2nd edn. McGraw-Hill, New York
Fink S (1982) Adventitious root primordia — the cause of abnormally broad xylem rays in hard- and softwood. IAWA Bull NS 3: 31–38
Halle F, Oldeman RAA (1975) An essay on the architecture and dynamics of growth of tropical trees. Penerbit University Press, Kuala Lumpur
Hejnowicz Z, Kurczynska EU (1987) Occurrence of circular vessels above axillary buds in tems of woody plants. Acta Soc Bot Pol 56: 415–419
Horn HS (1971) The adaptive geometry of trees. Princeton University Press, Princeton
Indig FE, Aloni R (1989) An experimental method for studying the differentiation of vessel endings. Ann Bot 64: 589–592
Isebrands JG, Larson PR (1977) Vascular anatomy of the nodal region in Populus deltoides Bartr. Am J Bot 64: 1066–1077
Jacobs WP (1952) The role of auxin in differentiation of xylem around a wound. Am J Bot 39: 301–309
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Kirschner H, Sachs T, Fahn A (1971) Secondary xylem reorientation as a special case of vascular tissue differentiation. Isr J Bot 20: 184–198
Kučera L (1971) Wundgewebe in der Eibe (Taxus baccata L.). Vierteljahrsschr Naturforsch Ges Zuer 116: 445–470
Küster E (1925) Pathologische Pflanzenanatomie, 3rd edn. Fischer, Jena
Larson PR, Isebrands JG (1978) Functional significance of the nodal constricted zone in Populus deltoides. Can J Bot 56: 801–804
Liphschitz N, Mendel Z (1987) Histological studies of Pinus halepensis stem xylem affected by Matsucoccus josephi (Homoptera: Margarodidae). IAWA Bull NS 8: 369–376
Luxová M (1986) The hydraulic safety zone at the base of barley roots. Planta 169: 465–470
Madar Z, Liphschitz N (1989) Histological studies of Cupressus sempervirens L. affected by Diplodia pinea f. sp. cupressi and Seiridium cardinale. IAWA Bull NS 10: 183–192
Millington WF, Chaney WR (1973) Shedding of shoots and branches. In: Kozlowski TT (ed) Shedding of plant parts. Academic Press, New York, pp 149–204
Neeff F (1914) Über Zellumlagerung. Ein Beitrag zur experimentellen Anatomie. Z Bot 6: 465–547
Neeff F (1922) Über polares Wachstum von Pflanzenzellen. Jahrb. Wiss Bot 61: 205–283
Phillips IDJ (1975) Apical dominance. Annu Rev Plant Physiol 26: 341–367
Roberts LW, Gahan PB, Aloni R (1988) Vascular differentiation and plant growth regulators. In: Timell TE (ed) Springer series in wood science. Springer, Berlin Heidelberg New York
Rubinstein B, Nagao MA (1976) Lateral bud outgrowth and its control by the apex. Bot Rev 42: 83–113
Sachs T (1968) On the determination of the pattern of vascular tissue in peas. Ann Bot 32: 781–790
Sachs T (1969) Polarity and the induction of organized vascular tissues. Ann Bot 33: 263–275
Sachs T (1975) The control of the differentiation of vascular networks. Ann Bot 39: 197–204
Sachs T (1981) The control of the patterned differentiation of vascular tissues. Adv Bot Res 9: 151–262
Sachs T, Cohen D (1982) Circular vessels and the control of vascular differentiation in plants. Differentiation 21: 22–26
Salleo S, Rosso R, LoGullo MA (1982) Hydraulic architecture of Vitis vinifera L. and Populus deltoides Bartr. 1-year-old twigs: II The nodal regions as “constriction zones” of the xylem system. G Bot Ital 116: 29–40
Salleo S, LoGullo MA, Siracusano L (1984) Distribution of vessel ends in stems of some diffuse- and ring-porous trees: the nodal regions as “safety zones” of the water conducting system. Ann Bot 54: 543–552
Sprengel F (1936) Über die Kropfkrankheit an Eiche, Kiefer und Fichte. Phytopathol Z 9: 583–635
Tsoumis G, Kezos N, Fanariotou I, Voulgaridis E, Passialis C (1988) Characteristics of briarwood. Holzforschung 42: 71–77
Tupper-Carey RM (1930) Observations on the anatomical changes in tissue bridges across rings through the phloem of trees. Leeds Phil Lit Soc Sci Sec 2: 86–94
Vöchting H (1892) Über Transplantion am Pflanzenkörper. Untersuchungen zur Physiologie und Pathologie. Laupp'schen, Tübingen
Vöchting H (1918) Untersuchungen zur experimentellen Anatomie und Pathologie des Pflanzenkörpers. II. Die Polarität der Gewächse. Laupp'schen, Tübingen
Wainwright SA (1988) Axis and circumference. The cylindrical shape of plants and animals. Harvard University Press, Cambridge
Wiebe HH, Greer RL, Van Alfen NK (1984) Frequency and grouping of essel endings in alfalfa (Medicago sativa) shoots. New Phytol 97: 583–590
Wloch W (1976) Cell events in cambium, connected with the formation and existence of a whirled cell arrangement. Acta Soc Bot Pol 45: 313–326
Zimmermann MH (1978) Structural requirements for optimal water conduction in tree stems. In: Tomlinson PB, Zimmermann MH (eds) Tropical trees as living systems. Cambridge University Press, Cambridge, pp 517–532
Zimmermann MH (1982) Functional xylem anatomy of angiosperm trees. In: Baas P (ed) New perspectives in wood anatomy. Nijhoff, The Hague, pp 59–70
Zimmermann MH (1983) Xylem structure and the ascent of sap. In: Timell TE (ed) Springer series in wood science. Springer, Berlin Heidelberg New York
Zimmermann MH, Sperry JS (1983) Anatomy of the palm Rhapis excelsa. IX. Xylem structure of the leaf insertion. J Arnold Arbor Harv Univ 64: 599–609
Zimmermann MH, Tomlinson PB (1965) Anatomy of the palm Rhapis excelsa I. Mature vegetative axis. J Arnold Arbor Harv Univ 46: 160–178 cd
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lev-Yadun, S., Aloni, R. Vascular differentiation in branch junctions of trees: circular patterns and functional significance. Trees 4, 49–54 (1990). https://doi.org/10.1007/BF00226240
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00226240