Summary
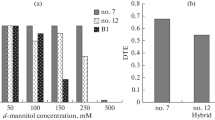
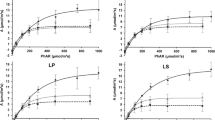
The factors affecting stomatal conductance (gs) of I-214 (Populus euramericana) and a hybrid poplar, Peace (P. koreana x P. trichocarpa), were examined in the field and under controlled environment conditions. Unusual opening of the stomata was observed with Peace leaves at all positions. Ontogenetic changes in gs were similar between these two poplar species in the light. However, the dark/light ratio of gs in Peace poplar varied from 0.58 to 1.23 with the insertion level while that of I-214 poplar was zero except for the third leaf from the top. The gs of I-214 poplar changed with time of the day, varying from 0.74 mol m-2s-1 in the morning to zero at night, while the gs of Peace poplar changed only from the minimum value of 0.23 mol m-2s-1 at night to the maximum of 0.48 mol m-2s-1 in the morning. Under severe water stress, below -1.5 MPa, which decreased the gs of I-214 poplar to almost zero, the gs of Peace poplar remained about onethird of that observed with well-watered leaves. Exposure to a relatively high concentration of O3 caused the gs of I-214 poplar to decrease nearly to zero but had no effect on the gs of Peace. Stomata of Peace poplar were not affected by ABA and the gs did not change even with 10-4 M ABA, while the gs of I-214 decreased to almost zero on the application of this concentration of ABA.
Similar content being viewed by others
References
Boyer JS (1970) Differing sensitivity of photosynthesis to low leaf water potentials in corn and soybean. Plant Physiol 46: 236–239
Carlson RW (1983) The effect of SO2 on photosynthesis and leaf resistance at varying conditions of CO2. Environ Pollut 30: 309–321
Ceulemans R, Impens I, Imler R (1988) Stomatal conductance and stomatal behavior in Populus clones and hybrids. Can J Bot 66: 1404–1414
Donkin ME, Wang TL, Martin ES (1983) An investigation into the stomatal behavior of a wilty mutant of Pisum sativum. J Exp Bot 34: 825–834
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Ann Rev Plant Physiol 33: 317–345
Farquhar GD, Dubbe DR, Raschke K (1978) Gain of the feedback loop involving carbon dioxide and stomata. Theory and measurements. Plant Physiol 62: 406–412
Fujinuma Y (1987) Evaluation of absorption capacity of vegetation for cleaning atmosphere. Res Report Natl Inst Environ Stud 108: 33–40
Furukawa A, Katase M, Ushijima T, Totsuka T (1983) Inhibition of photosynthesis of poplar species by ozone. J Jpn For Soc 65: 321–326
Gaastra P (1959) Photosynthesis of crop plants as influenced by light, carbon dioxide, temperature, and stomatal resistance. Meded Landbouwhogesch Wageningen 59: 1–68
Kaplan A, Gale J (1972) Effect of sodium chloride salinity on the water balance of Atriplexhalimus. Aust J Biol Sci 25: 895–903
Kondo N, Sugahara K (1978) Changes in transpiration rate of SO2-resistant and -sensitive plants with SO2 fumigation and the participation of abscisic acid. Plant Cell Physiol 19: 365–373
Lancaster JE, Mann JD, Porter NG (1977) Ineffectiveness of abscisic acid in stomatal closure of yellow lupin, Lupinus luteus var. Weiko III. J Exp Bot 28: 184–191
Muchow RC, Ludlow MM, Fischer MJ, Myers RJK (1980) Stomatal behaviour of kenaf and sorghum in a semiarid tropical environment. I. During the night. Aust J Plant Physiol 7: 609–619
Nobel PS (1983) Biophysical Plant Physiology and Ecology. WH Freeman, San Francisco
Pemadasa MA (1981) Abaxial and adaxial stomatal behaviour and responses to fusicoccin on isolated epidermis of Commelina cornmunis. New Phytol 89: 373–384
Quarrie SA (1982) Droopy. A wilty mutant of potato deficient in abscisic acid. Plant Cell Environ 5: 23–26
Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA (1965) Sap pressure in vascular plants. Science 148: 339–345
Sharpe PJH (1973) Adaxial and abaxial stomatal response of cotton in the field. Agron J 65: 570–574
Slatyer RO (1967) Plant-water relationships. Academic Press, London
Slavik B (1974) Methods of studying plant water relations. Springer, Berlin Heidelberg New York
Tal M (1966) Abnormal stomatal behavior in wilty mutants of tomato. Plant Physiol 41: 1387–1391
Tal M, Nevo Y (1973) Abnormal stomatal behavior and root resistance, and hormonal imbalance in three wilty mutants of tomato. Biochem Genet 8: 291–300
Tal M, Imber D, Itai C (1970) Abnormal stomatal behavior and hormonal imbalance in flacca, a wilty mutant of tomato. I. Root effect and kinetin-like activity. Plant Physiol 46: 367–372
Winner WE, Mooney HA (1980) Ecology of SO2 resistance. II. Photosynthetic changes of shrubs in relation to SO2 absorption and stomatal behavior. Oecologia 44: 296–302
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Furukawa, A., Park, SY. & Fujinuma, Y. Hybrid poplar stomata unresponsive to changes in environmental conditions. Trees 4, 191–197 (1990). https://doi.org/10.1007/BF00225315
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00225315