Abstract
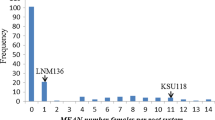
The mapping of resistance toMeloidogyne chitwoodi derived from Solarium bulbocastanum is reported. A population suitable for mapping was developed as follows. A somatic hybrid of nematode-resistant S. bulbocastanum and cultivated tetraploid potato was produced. This was backcrossed to tetraploid potato, and a single resistant BC1 was selected and backcrossed again to the same recurrent tetraploid parent. The mapping population consisted of 64 BC2 progeny scored for restriction fragment length polymorphic (RFLP) markers and 62 of these were evaluated for the reproductive efficiency of race 1 of M. chitwoodi. Forty-eight polymorphic RFLP markers, originally derived from tomato and mapped in diploid cultivated potato, were assigned to 12 chromosomes of S. bulbocastanum. Of the 62 progeny screened for nematode resistance, 18 were non-hosts and four were poor hosts. The rest were highly susceptible (good hosts). Analysis of the resistance (including non-hosts and poor hosts) as both a qualitative trait and as a meristic trait on which QTL analysis was applied supported the same genetic hypothesis. Genetic control was localized solely to factor(s) lying at one end of chromosome 11. The level of expression of resistance in the S. bulbocastanum parent and the resistant portion of the BC2 was essentially the same. This fact, together with the highly significant LOD scores for one end of the chromosome-11 marker array, supports a genetic model equivalent to monogenic dominant control.
Similar content being viewed by others
References
Austin S, Pohlman JD, Brown CR, Mojtahedi H, Santo GS, Douches D, Helgeson JP (1993) Interspecific somatic hybridization between Solanum tuberosum L. and S. bulbocastanum DUN. as a means of transferring nematode resistance. Am Potato J 70:485–495
Azanza F, Young TE, Kim D, Tanksley, SD, Juvik JA (1994) Characterization of the effect of introgressed segments of chromosome 7 and 10 from Lycopersicon chmielewskii on tomato soluble solids, pH, and yield. Theor Appl Genet 87:965–972
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Bonierbale MW, Plaisted RL, Tanksley SD (1988) RFLP maps based on a common set of clones reveal modes of chromosomal evolution in potato and tomato. Genetics 120:1095–1103
Bonierbale MW, Plaisted RL, Tanksley SD (1990) Application of restriction fragment length polymorphisms and genetic mapping to potato breeding. In: Molecular methods for potato improvement. Report of the Planning Conference on “Application of Molecular Techniques to Potato Germ Plasm Enhancement.” International Potato Center, Lima, Peru, pp149–159
Bonierbale MW, Plaisted RL, Pineda O, Tanksley SD (1994) QTL analysis of trichome-mediated insect resistance in potato. Theor Appl Genet 87:973–987
Brown CR, Mojtahedi H, Santo GS (1989) Comparison of the reproductive efficiency of Meloidogyne chitwoodi on Solanum bulbocastanum in soil and in vitro tests. Plant Dis 73:957–959
Brown, CR, Mojtahedi H, Santo GS (1991) Resistance to Columbia root-knot nematode in Solanum spp. and in hybrids of S. hougasii with cultivated tetraploid potato. Am Potato J 68: 445–452
Brown CR, Mojtahedi H, Santo GS, Austin-Phillips S (1994) Enhancing resistance to root-knot nematodes derived from wild Solanum species in potato germ plasm. In: Zehnder GW, Powelson ML, Jansson RK, Raman KV (eds) Advances in potato pest biology and management. Am Phytopathal Soc, Minneapolis, Minnesota, pp 426–438
Brown CR, Mojtahedi H, Santo GS (1995) Introgression of resistance to Columbia and Northern root-knot nematodes from Solanum bulbocastanum into cultivated potato. Euphytica 83:71–78
Debener T, Salamini F, Gebhardt C (1990) Phylogeny of wild and cultivated Solanum species based on nuclear restriction fragment length polymorphisms (RFLPs). Theor Appl Genet 79:360–368
Evans K, Trudgill D (1992) Pest aspects of potato production part 1. The nematode pests of potatoes. In: Harris P (ed) The potato crop. Chapman and Hall, London, pp 438–475
Freyre R, Warnke S, Sosinski B, Douches DS (1994) Quantitative trait locus analysis of tuber dormancy in diploid potato (Solanum spp.) Theor Appl Genet 89:474–480
Gebhardt C, Ritter E, Debener T, Schachtschabel U, Walkemeier B, Uhrig H, Salamini F (1989) RFLP analysis and linkage mapping in Solanum tuberosum. Theor Appl Genet 78:65–75
Gebhardt C, Ritter E, Barone A, Debener T, Walkemeier B, Schachtschabel U, Kaufman H, Thompsom RD, Bonierbale MW, Tanksley SD, Salamini F (1991) RFLP maps of potato and their alignment with the homoeologous tomato genome. Theor Appl Genet 83:49–57
Gebhardt C, Mugniery D, Ritter E, Salamini F, Bonnel E (1993) Identification of RFLP markers closely linked to the H1 gene conferring resistance to Globodera rostochiensis in potato. Theor Appl Genet 85:541–544
Hoyman WG (1974) Reaction of Solanum tuberosum and Solanum species to Meloidogyne hapla. Am Potato J 51:281–286
Hussey RS, Barker KR (1973) A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis Rep 57:1025–1028
Kraft RJ, Tardiff J, Krauter KS, Leinwand, LA (1988) Using miniprep plasmid DNA for sequencing double-stranded templates with sequenase. Biotechniques 6:544–547
Kreike CM, de Koning JRA, Vinke JH, van Ooijen JW, Gebhardt C, Stiekema WJ (1993) Mapping of loci involved in quantitatively inherited resistance to the potato cyst-nematode Globodera rostochiensis pathotype Ro1. Theor Appl Genet 87:464–470
Lander E, Green P, Abrahamson J, Barlow A, Daley M, Lincoln S, Newburg L (1987) MAPMAKER: An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Masuelli RW, Tanimoto EY, Brown CR, Comai L (1995) Irregular meiosis in a somatic hybrid between Solanum bulbocastanum and S. tuberosum detected by species-specific PCR markers and cytological analysis. Theor Appl Genet 91:801–809
Oostenbrink M (1966) Major characteristics of the relation between nematodes and plants. Meded Landbouwhogeschool, Wageningen 66:3–46
Paterson A, Lander E, Lincoln S, Hewitt J, Peterson S, Tanksley S (1988) Resolution of quantitative traits into Mendelian factors using a complete RFLP linkage map. Nature 335:721–726
Pineda O, Bonierbale MW, Plaisted RL, Brodie BB, Tanksley SD (1993) Identification of RFLP markers linked to the H1 gene conferring resistance to the potato cyst nematode Globodera rostochiensis. Genome 36:152–156
Pinkerton JN, Mojtahedi H, Santo GS (1987) Reproductive efficiency of Pacific Northwest populations of Meloidogyne chitwoodi on alfalfa. Plant Dis 71:345–348
Salentjin EMJ, Arens-De Reuver MJB, Lange W, De Bock ThSM, Stiekema WJ, Klein-Lankhorst RM (1995) Isolation and characterization of RAPD-based markers linked to the beet cyst nematode resistance locus (Hs1 pat-1) on chromosome 1 of B. patellaris. Theor Appl Genet 90:885–891
Sasser JN, Carter CC, Hartman KM (1984) Standardization of host suitability studies and reporting of resistance to root-knot nematodes. Crop Nematode Res Control Proj, NCSU/USAID, Dept of Plant Pathol, NCSU, Box 7616, Raleigh, North Carolina 27695, USA
Tanksley SD (1993) Mappingpolygenes. Annu Rev Genet 27:205–233
Tanksley SD, Ganal MW, Prince JP, de Vicente MC, Bonierbale MW, Broun P, Fulton TM, Giovannoni JJ, Grandillo S, Martin GB, Messeguer R, Miller JC, Miller L, Paterson AH, Pineda O, Roder MS, Wing RA, Wu W, Young ND (1992) High-density molecular maps of the tomato and potato genomes. Genetics 132:1141–1160
Uphoff H, Wricke G (1992) Random amplified polymorphic DNA (RAPD) markers in sugar beet (Beta vulgaris L.): mapping the genes for nematode resistance and hypocotyl colour. Plant Breed 109:168–171
Williamson VM, Ho J-Y, Wu FF, Miller N, Kaloshian I (1994) A PCR-based marker tightly linked to the nematode resistance gene, Mi, in tomato. Theor Appl Genet 87:757–763
Author information
Authors and Affiliations
Additional information
Communicated by H. F. Linskens
Rights and permissions
About this article
Cite this article
Brown, C.R., Yang, C.P., Mojtahedi, H. et al. RFLP analysis of resistance to Columbia root-knot nematode derived from Solanum bulbocastanum in a BC2 population. Theoret. Appl. Genetics 92, 572–576 (1996). https://doi.org/10.1007/BF00224560
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00224560