Abstract
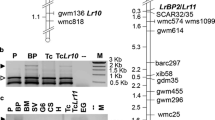
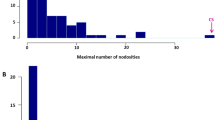
Accessions of the wild tomato species L. peruvianum were screened with a root-knot nematode population (557R) which infects tomato plants carrying the nematode resistance gene Mi. Several accessions were found to carry resistance to 557R. A L. peruvianum backcross population segregating for resistance to 557R was produced. The segregation ratio of resistant to susceptible plants suggested that a single, dominant gene was a major factor in the new resistance. This gene, which we have designated Mi-3, confers resistance against nematode strains that can infect plants carrying Mi. Mi-3, or a closely linked gene, also confers resistance to nematodes at 32°C, a temperature at which Mi is not effective. Bulked-segregant analysis with resistant and susceptible DNA pools was employed to identify RAPD markers linked to this gene. Five-hundred-and-twenty oligonucleotide primers were screened and two markers linked to the new resistance gene were identified. One of the linked markers (NR14) was mapped to chromosome 12 of tomato in an L. esculentum/L. pennellii mapping population. Linkage of NR14 and Mi-3 with RFLP markers known to map on the short arm of chromosome 12 was confirmed by Southern analysis in the population segregating for Mi-3. We have positioned Mi-3 near RFLP marker TG180 which maps to the telomeric region of the short arm of chromosome 12 in tomato.
Similar content being viewed by others
References
Ammati M, Thomason IJ, McKinney HE (1986) Retention of resistance to Meloidogyne incognita in Lycopersicon genotypes at high soil temperature. J. Nematol 18:491–495
Cap GP, Roberts PA, Thomason IJ (1993) Inheritance of heat-stable resistance to Meloidogyne incognita in Lycopersicon peruvianum and its relationship to gene Mi. Theor Appl Genet 85:777–783
Dropkin VH (1969) The necrotic reaction of tomatoes and other hosts resistant to Meloidogyne: reversal by temperature. Phytopathology 59:1632–1637
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19:1349
Eisenback JD, Triantaphyllou HH (1991) Root-knot nematodes: Meloidogyne species and races. In: Nickel WR (ed) Manual of agricultural nematology. Marcel Dekker, New York, pp 191–274
Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction fragments to high specific activity. Anal Biochem 132:6–13
Ho JY, Weide R, Ma HM, van Wordragen MF, Lambert KN, Koornneef M, Zabel P, Williamson VM (1992) The root-knot nematode resistance gene (Mi) in tomato: construction of a molecular linkage map and identification of dominant cDNA markers in resistant genotypes. The Plant J 2:971–982
Jarquin-Barberena H, Dalmasso A, deGuiran G, Cardin MC (1991) Acquired virulence in the plant parasitic nematode Meloidogyne incognita. 1. Biological analysis of the phenomenon. Rev Nematol 14:261–275
Lambert KN, Tedford EC, Caswell EP, Williamson VM (1992) A system for continuous production of root-knot nematode juveniles in hydroponic culture. Phytopathology 82:512–515
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Lobo M, Navarro R, Munera G (1988) Meloidogyne incognita and M. javanica resistance in Lycopersicon species. Tomato Genet Coop 38:31
Mai WF (1985) Plant-parasitic nematodes: their threat to agriculture. In: Sasser, Carter (eds) An advanced treatise on Meloidogyne, North Carolina State University Graphics, pp 11–17
Medina-Filho M, Tanksley SD (1983) Breeding for nematode resistance. In: Evans DA, Sharp WR, Ammirato PV, Yamada Y (eds) Handbook of plant cell culture, vol. 1 Macmillan, New York, pp 904–923
Messeguer R, Ganal M, de Vicente MC, Young ND, Bolkan H, Tanksley SD (1991) High-resolution RFLP map around the root-knot nematode resistant gene (Mi) in tomato. Theor Appl Genet 82:529–536
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Omwega CO, Thomason IJ, Roberts PA (1988) A non-destructive technique for screening bean germ plasm for resistance to Meloidogyne incognita. Plant Dis 72:970–972
Rick CM (1963) Barriers to interbreeding in Lycopersicon peruvianum. Evolution 17:216–232
Rick CM, Yoder JI (1988) Classical and molecular genetics of tomato: highlights and perspectives. Annu Rev Genet 22:281–300
Riggs RD, Winstead NN (1959) Studies on resistance in tomato to root-knot nematodes and on the occurrence of pathogenic biotypes. Phytopathology 49:716–724
Roberts PA, Thomason IJ (1986) Variability in reproduction of isolates of Meloidogyne incognita and M. javanica on resistant tomato genotypes. Plant Dis 70:547–551
Roberts PA, Dalmasso A, Cap GB, Castagnone-Sereno P (1990) Resistance in Lycopersicon peruvianum to isolates of Mi genecompatible Meloidogyne populations. J Nematol 22:585–589
Smith PG (1944) Embryo culture of a tomato species hybrid. Proc Am Soc Hort Sci 44:413–416
Tanksley SD, Ganal MW, Prince JP, de Vicente MC, Bonierbale MW, Broun P, Fulton TM, Giovannoni JJ, Grandillo S, Martin GB, Messeguer R, Miller JC, Miller L, Paterson AH, Pineda O, Roder MS, Wing RA, Wu RA, Wu W, Young ND (1992) High-density molecular linkage maps of the tomato and potato genomes. Genetics 132:1141–1160
Triantaphyllou AC (1987) Genetics of nematode parasitism on plants. In: Veech JA, Dickson DW (eds) Vistas on nematology. Published by Society of Nematologists, pp 354–363
Williams GGK, Kubelik AR, Livak KJ, Rafalski JA, Tingy SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Williamson VM, Colwell G (1991) Acid phosphatase-1 from nematode-resistant tomato: isolation and characterization of the gene. Plant Physiol 97:139–146
Williamson VM, Ho JY, Wu FF, Miller N, Kaloshian I (1994) A PCR-based marker tightly linked to the nematode resistance gene, Mi, in tomato. Theor Appl Genet 87:757–763
Author information
Authors and Affiliations
Additional information
Communicated by M. Koornneef
Rights and permissions
About this article
Cite this article
Yaghoobi, J., Kaloshian, I., Wen, Y. et al. Mapping a new nematode resistance locus in Lycopersicon peruvianum . Theoret. Appl. Genetics 91, 457–464 (1995). https://doi.org/10.1007/BF00222973
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00222973