Abstract
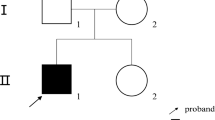
Lesch-Nyhan syndrome caused by a complete deficiency of hypoxanthine guanine phosphoribosyltransferase (HPRT) is the result of a heterogeneous group of germ line mutations. Identification of each mutant gene provides valuable information as to the type of mutation that occurs spontaneously. We report here a newly identified HPRT mutation in a Japanese patient with Lesch-Nyhan syndrome. This gene, designated HPRT Tokyo, had a single nucleotide change from G to A, as identified by sequencing cDNA amplified by the polymerase chain reaction. Allele specific oligonucleotide hybridization analysis using amplified genomic DNA showed that the mutant gene was transmitted from the maternal germ line. This mutation would lead to an amino acid substitution of Asp for Gly at the amino acid position 140 located within the putative 5-phosphoribosyl-1-pyrophosphate (PRPP) binding region. Missense mutations in human HPRT deficient patients thus far reported tend to accumulate in this functionally active region. However, a comparison of the data suggested that both missense and synonymous mutations can occur at any coding sequence of the human germ line HPRT gene, but that a limited percentage of all the missense mutations cause disease. The probability that a mutation will cause disease tends to be higher when the missense mutation is within a functionally important sequence.
Similar content being viewed by others
References
Chou PY, Fasman GD (1978) Empirical predictions of protein conformation. Annu Rev Biochem 47:251–276
Craig SP, McKerrow JH, Newport GR, Wang CC (1988) Analysis of cDNA encoding the hypoxanthine-guanine phosphoribosyltransferase (HPRTase) of Schistosoma mansoni; a putative target for chemotherapy. Nucleic Acids Res 16:7087–7101
Davidson BL, Palella TD, Kelley WN (1988) Human hypoxanthine guanine phosphoribosyltransferase: a single nucleotide substitution in cDNA clones isolated from a patient with Lesch-Nyhan syndrome (HPRT Midland) Gene 68:85–91
Fujimori S, Hidaka Y, Davidson BL, Palella TD, Kelley WN (1988) Identification of a single nucleotide change in a mutant gene for hypoxanthine guanine phosphoribosyltransferase (HPRT Ann Arbor). Hum Genet 79:39–43
Fujimori S, Kamatani N, Nishida Y, Ogasawara N, Akaoka I (1990) Hypoxanthine guanine phosphoribosyltransferase deficiency: nucleotide substitution causing Lesch-Nyhan syndrome identified for the first time among Japanese. Hum Genet 84:483–486
Gibbs RA, Nguyen PN, McBride LJ Koepf SM, Caskey CT (1989) Identification of mutations leading to Lesch-Nyhan syndrome by automated direct DNA sequencing in vitro amplified cDNA. Proc Natl Acad Sci USA 86:1919–1923
Gibbs RA, Nguyen PN, Edwards AL, Civitello AB, Caskey CT (1990) Multiplex DNA deletion detection and exon sequencing of the hypoxanthine phosphoribosyltransferase gene in Lesch-Nyhan families. Genomics 7:235–244
Hershey HV, Taylor MW (1986) Nucleotide sequence and deduced amino acid sequences of Escherichia coli adenine phosphoribosyltransferase and comparison with other analogous enzymes. Gene 43:287–293
Hopp TP, Woods KR (1981) Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci USA 78:3824–3828
Kelley WN, Rosenbloom FM, Henderson JF, Seegmiller JE (1967) A specific enzyme defect in gout associated with overproduction of uric acid. Proc Natl Acad Sci USA 57:1735–1739
King A, Melton DW (1987) Characterization of cDNA clones for hypoxanthine-guanine phosphoribosyltransferase from the human malarial parasite, Plasmodium falciparum: comparisons to the mammalian gene and protein. Nucleic Acids Res 15:10469–10481
Konecki DS, Brennand J, Fuscoe JC, Caskey CT, Chinault AC (1982) Hypoxanthine-guanine phosphoribosyltransferase gene of mouse and Chinese hamster: construction and sequences analysis of cDNA recombinants. Nucleic Acids Res 10:6763–6775
Seegmiller JE, Rosenbloom FM, Kelley WN (1967) Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science 155:1682–1684
Showalter RE, Silverman MR (1990) Nucleotide sequence of a gene, hpt, for hypoxanthine phosphoribosyltransferase from Vibrio harveyi, Nucleic Acids Res 18:4621
Stout JT, Caskey CT (1989) Hypoxanthine phosphoribosyltransferase: the Lesch-Nyhan syndrome and gouty arthritis. In: Seriver CR, et al (eds) The metabolic basis of inherited disease, 6th edn. McGraw-Hill, New York, pp 1007–1028
Wilson JM, Tarr GE, Mahoney WC, Kelley WN (1982) Human hypoxanthine-guanine phosphoribosyltransferase: complete amino acid sequence of the erythrocyte enzyme. J Biol Chem 257:10978–10985
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fujimori, S., Tagaya, T., Kamatani, N. et al. A germ line mutation within the coding sequence for the putative 5-phosphoribosyl-1-pyrophosphate binding site of hypoxanthine-guanine phosphoribosyltransferase (HPRT) in a Lesch-Nyhan patient: missense mutations within a functionally important region probably cause disease. Hum Genet 90, 385–388 (1992). https://doi.org/10.1007/BF00220464
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00220464