Summary
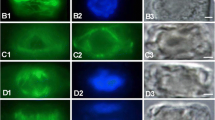
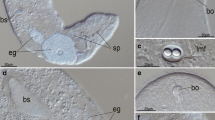
Immunofluorescence and electron microscopy were used to analyze the relationships between the organization of collagen fibrils in elasmoid scales, and the orientation of microtubules and actin microfilaments in the scleroblasts producing this collagenous stroma. Attention was focused on the basal plate of the scales because of the highly ordered three-dimensional arrangement of the collagen fibrils in superimposed plies forming an acellular plywood-like structure. The collagen fibrils are synthesized by the scleroblasts forming a monolayered pseudo-epithelium, the hyposquama, at the lowest surface of the scale. Fully developed scales with a low collagen deposition rate were compared with regenerating scales active in fibrillogenesis. When an ordered array of the collagen fibrils is found, the innermost collagen fibrils are coaligned with microtubules and actin microfilaments. Thus, because of this coalignment, microtubules and actin microfilaments of the hyposquamal scleroblasts are subjected to consecutive alterations during the formation of the plies of the basal plate. The sequence of events when the collagen fibrils change their direction from one ply to the other in the basal plate is deduced from immunofluorescence and phase-contrast-microscopic observations. During the formation of the orthogonal plywood-like structure in the regenerating scales, first microtubules may change their curse with a rotating angle of about 90°; then, actin microfilaments are disorganized and reorganized by interacting mechanically with the microtubules with which they are coaligned. Collagen fibrils are synthesized in a direction that is roughly perpendicular to that of the preceding ply. The unknown signals inducing the change in direction of the cytoskeleton may be transmitted throughout the hyposquama via gap junctions.
Similar content being viewed by others
References
Allizard F, Zylberberg L (1982) A technical improvement for sectioning hard lamined fibrous tissues for electron microscopic studies. Stain Technol 57:335–339
Bertin L (1958) Ecailles et sclerifications dermiques. In: Grassé P-P (ed) Traité de Zoologie. 13 Poissons. Masson, Paris, pp 53–129
Birk DE, Trelstad RL (1984) Extracellular components in matrix morphogenesis: collagen fibrils, bundle, lamellar formation by corneal fibroblasts. J Cell Biol 99:2024–2033
Birk DE, Trelstad RL (1985) Fibroblasts compartmentalize the extracellular space to regulate and facilitate collagen fibril, bundle, and macroaggregate formation. In: Extracellular matrix: Structure and function. Alan R. Liss Inc, New York, pp 373–382
Birk DE, Trelstad RL (1986) Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol 103:231–240
Bissell MJ, Hall HG, Perry G (1982) How does the extracellular matrix direct gene expression. J Theor Biol 99:31–68
Bouligand Y (1972) Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell 4:189–217
Bouligand Y, Denèfle JP, Lechaire JP, Maillard M (1985) Twisted architecture in cell-free assembled collagen gels: Study of collagen substrates used for cultures. Biol Cell 54:143–162
Brown GA, Wellings SR (1969) Collagen formation and calcification in teleost scales. Z Zellforsch 93:571–582
Byers HR, Fujiwara K (1982) Stress fibers in cells in situ: Immunofluorescence with antiactin, antimyosin, and anti-alphaactinin. J Cell Biol 93:804–811
Byers HR, Fujiwara K, Porter K (1980) Visualization of microtubules of cells in situ by indirect immunofluorescence. Proc Natl Acad Sci USA 77:6657–6661
Dane PJ, Tucker JB (1986) Supracellular microtubule alignements in cell layers associated with the secretion of certain fish scales. J Cell Sci Suppl 5:273–291
Dehm P, Prockop DJ (1972) Time lag in the secretion of collagen by matrix-free tendon cells and inhibition of the secretory process by colchicine and vinblastine. Biochim Biophys Acta 264:375–382
Ehrlich HP, Ross R, Bornstein P (1974) Effects of antimicrotubular agents on the secretion of collagen. A biochemical and morphological study. J Cell Biol 62:390–405
Escaig J, Nicolas G (1976) Cryofracture de matériel biologique réalisée à très basse température et en ultravide. CR Acad Sci Paris, Série D 283:1245–1248
Frietsche RA, Bailey CF (1980) The histology and calcification of regenerating scales in the blackspotted minnow, Fundulus olivaceus (Storer). J Fish Biol 16:693–700
Giraud MM, Castanet J, Meunier JF, Bouligand Y (1978) The fibrous structure of coelacanth scales: a twisted “plywood”. Tissue Cell 10:671–686
Hay ED (1983) Cell and extracellular matrix: their organization and mutual dependence. In: Satir BH (ed) Modern Cell Biology. AR Liss Inc, New York, Vol 2, pp 509–548
Hay ED, Dodson JW (1973) Secretion of collagen by corneal epithelium. I. Morphology of the collagenous products produced by isolated epithelia grown on frozen-killed lens. J Cell Biol 57:190–213
Kilmartin JV, Wright B, Milstein L (1982) Rat monoclonal antitubulin antibodies derived by using non secreting rat cell line. J Cell Biol 93:576–582
Klaatsch H (1894) Über die Herkunft des Scleroblasten. Ein Beitrag von Osteogenese. Morphol Jahrb 21:153–240
Lane B, Bartek J, Purkis PW, Leight IM (1985) Keratin antigenes in differentiating skin. Ann NY Acad Sci 455:241–258
Meunier FJ (1984) Spatial organization and mineralization of the basal plate of elasmoid scales in Osteichthyans. Am Zool 24:953–964
Meunier FJ, Castanet J (1982) Organisation spaciale des fibres de collagene de la plaque basale des écailles des Téléostéens. Zool Scr 11:141–153
Onozato H, Watabe N (1979) Studies on fish scale formation and resorption. III. Fine structure and calcification of the fibrillary plates of the scales in Carassius auratus (Cypriniformes: Cyprinidae). Cell Tissue Res 201:409–422
Opas M (1987) The transmission of forces between cells and their environment. In: Bereiter-Hahn J, Anderson OR, Reif W-E (eds) Cytomechanics. Springer, Berlin Heidelberg New York, pp 273–286
Reynolds ES (1963) The use of lead citrate of high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Schönbörner AA, Boivin G, Baud CA (1979) The mineralization processes in teleost fish scales. Cell Tissue Res 202:203–212
Singer II (1979) The fibronexus: A transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell 16:675–685
Sire JY, Géraudie J (1984) Fine structure of regenerating scales and their associated cells in the cichlid Hemichromis bimaculatus (Gill). Cell Tissue Res 237:537–547
Sire JY, Meunier FJ (1981) Structure et minéralisation de l'écaille d'Hemichromis bimaculatus (Téléostéen, Perciforme, Cichlide) Arch Zool Exp Gén 122:133–150
Trelstad RL (1982) The bilaterally asymetrical architecture of the submammalian corneal stroma resembles a cholesteric liquid crystal. Dev Biol 92:133–134
Trelstad RL, Birk DE (1984) Collagen fibril assembly at the surface of polarized cells. In: Trelstad RL (ed) The role of extracellular matrix in development. Alan R Liss Inc, New York, pp 513–543
Unemori E, Werb Z (1986) Reorganization of polymerized actin: a possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J Cell Biol 103:1021–1031
Waterman RE (1970) Fine structure of scale development in the teleost, Brachydanio rerio. Anat Rec 168:361–380
Wehland J, Willingham MC, Sandoval IV (1983) A rat monoclonal antibody reacting specifically with tyrosinated form of alpha tubulin. I. Biochemical characterization, effects on microtubule polymerization in vitro, and microtubule polymerization and organization in vivo. J Cell Biol 97:1467–1475
Weiss P (1958) Cell contact. Int Rev Cytol 7:391–423
Wohlfahrt-Botterman K-E (1987) Dynamic organization and force production in cytoplasmic strands. In: Bereiter-Hahn J, Anderson, OR, Reif W-E (eds) Cytomechanics, Springer, Berlin Heidelberg New York, pp 154–168
Zylberberg L, Nicolas G (1982) Ultrastructure of scales in a teleost (Carassius auratus L.) after use of rapid freeze-fixation and freeze-substitution. Cell Tissue Res 223:349–367
Author information
Authors and Affiliations
Additional information
This work is dedicated to the memory of Jacques Escaig
Rights and permissions
About this article
Cite this article
Zylberberg, L., Bereiter-Hahn, J. & Sire, J.Y. Cytoskeletal organization and collagen orientation in the fish scales. Cell Tissue Res. 253, 597–607 (1988). https://doi.org/10.1007/BF00219750
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00219750