Summary
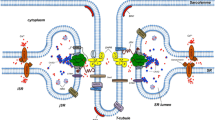
The disposition of surface invaginations (clefts, Z and T tubules) and of the sarcoplasmic reticulum has been examined by electron microscopy at three accelerating voltages (100, 200 and 1000 kV) and by phase-contrast light microscopy in crustacean muscles infiltrated by the “Golgi stain.” In long-sarcomere, tonic type fibers, an extensive system of invaginating clefts has been observed, along with both Z and T tubules. Z and T tubules form interconnections with each other, but only T tubules form specific contacts with the sarcoplasmic reticulum, which in these fibers forms an extended and continuously fenestrated network. In short-sarcomere, phasic type fibers, a ladder-like disposition of an abundant T network is found. Z tubules are absent in these fibers. The sarcoplasmic reticulum forms more frequent junctions with flattened areas of T tubules and with clefts, but has less extensive free surfaces than in the long-sarcomere fibers.
Similar content being viewed by others
References
Atwood HL (1973) An attempt to account for diversity of crustacean muscles. Am Zool 13:357–378
Atwood HL (1976) Organization and synaptic physiology of crustacean neuromuscular systems. Progr Neurobiol 7:291–391
Atwood HL, Hoyle F, Smyth T (1965) Mechanical and electrical responses of singly innervated crab-muscle fibers. J Physiol Lond 180:449–482
Bailey CH, Peachey LD (1975) High voltage electron microscopy of the T system in slow fibers of the frog cruralis muscle. Procs 33rd Ann Meeting EMSA, Las Vegas, 554–555
Brandt PN, Reuben JP, Girardier L, Grundfest H (1965) Correlated morphological and physiological studies on isolated single muscle fibers. I. Fine structure of the crayfish muscle fiber. J Cell Biol 245:233–260
Cohen MJ, Hess A (1967) Fine structural differences in “fast” and “slow” muscle fibers of the crab. Am J Anat 121:285–304
Crowe LM, Baskin RJ (1981) Activation of the contractile system in crustacean muscle: Ultrastructural evidence for the role of the T-system. Tissue & Cell 13:153–164
Dauber W (1979) Zur fasertypischen Morphologie und Funktion der Triaden im Skeletmuskel des Frosches (Rana esculenta). Z mikroskop-anat Forsch. Leipzig 93:512–536
Dewey MM, Levine RJC, Colflesh DE (1973) Structure of Limulus striated muscle. The contractile apparatus at various sarcomere lengths. J Cell Biol 58:574–593
Dorai-Raj BS (1964) Diversity of crab muscle fibers innervated by a single motor axon. J Cell Comp Physiol 64:41–54
Eagles DA, Riordan GP (1980) The occurrence of dyads between both Z and T tubules and sarcoplasmic reticulum in the region of overlap of thin and thick myofilaments in horseshoe crab muscle. Trans Am Micros Soc 99:78–93
Eagles DA, DeAndre GA, Riordan CP (1982) The T-axial membrane system in striated muscle of the horseshoe crab. Tissue and Cell 14:531–540
Eastwood EA, Franzini-Armstrong C, Peracchia C (1982) Freezefracture of crayfish muscle fibers. J Muscle Res Cell Motil 3:273–294
Eguilar de M, Ferraguti M (1980) Architecture of the T system in helical paramyosin muscles of an annelid (Branchiobdella). Tissue and Cell 12:739–747
Eguilar de M, Valvassori R, Lanzavecchia G (1980) Discontinuity of SR in the mid-sarcomere region in flight muscle of dragonflies. Tissue and Cell 12:749–759
Fahrenbach WH (1963) The sarcoplasmic reticulum of striated muscle of a cyclopoid copepod. J Cell Biol 17:629–640
Fahrenbach WH (1964) A new configuration of the sarcoplasmic reticulum. J Cell Biol 22:477–481
Fahrenbach WH (1967) The fine structure of fast and slow crustacean muscles. J Cell Biol 35:69–97
Forbes MS, Rubio R, Sperelekis N (1972) Tubular system of Limulus myocardial cells investigated by use of electron-opaque tracers and hypertonicity. J Ultrastr Res 39:580–597
Forbes MS, Hawkey LA, Sperelakis N (1984) The transverse-axial tubular system (TATS) of mouse myocardium: its morphology in the developing and adult animal. Am J Anat 170:143–162
Fourtner CR, Sherman RG (1972) A light and electron microscopic examination of muscles in the walking legs of the horseshoe crab, Limulus polyplemus (L). Can J Zool 50:1447–1455
Franzini-Armstrong C (1970) Natural variability in the length of thin and thick filaments in single fibers from a crab, Portunus depurator. Cell Sci 6:559–592
Franzini-Armstrong C (1973) Studies of the triad. IV Structure of the junction in frog slow fibers. J Cell Biol 56:120–128
Franzini-Armstrong C, Peachey LD (1982) A modified Golgi black reaction method for light and electron microscopy. J Histochem Cytochem 30:99–105
Franzini-Armstrong C, Eastwood AE, Peachey LD (1978) A new view of Veratti's “reticulum” in crustacean muscle fibers. J Cell Biol 79:330a
Gilai A, Parnas I (1972) Electromechanical coupling in tubular muscle fibers. I The organization of tubular muscle fibers in the scorpion Leisurus quinquestriatus. J Cell Biol 52:626–638
Hoyle G (1983) Muscles and their neural control. Wiley, New York
Hoyle G, McNeill PA (1968) Correlated physiological and ultrastructural studies on specialized muscles. Ib. Ultrastructure of white and pink fibers of the levator of the eyestalk of Podophthalmus vigil (Weber). J Exp Zool 167:487–522
Hoyle G, McNeill PA, Selverston AI (1973) Ultrastructure of barnacle giant muscle fibers. J Cell Biol 56:74–91
Huxley AF, Taylor RE (1958) Local activation of striated muscle fibers. J Physiol 144:426–441
Jahromi SS, Atwood HL (1967) Ultrastructural features of crayfish phasic and tonic muscle fibers. Can J Zool 45:601–606
Jahromi SS, Atwood HL (1969) Correlation of structure, speed of contraction and total tension in fast and slow abdominal muscles of the lobster (Homarus americanus). J Exp Zool 171:25–38
Kawaguti SA, Kawishima Y (1969) Electron microscopy of the long sarcomere muscle of the spider leg. Biol J Okayama Univ 15:73–86
Leyton RA, Sonnenblick EH (1971) Cardiac muscle of the horse shoe crab, Limulus polyphemus. I Ultrastructure. J Cell Biol 48:101–119
McNeill P, Burrows M, Hoyle G (1972) Fine structure of muscles controlling the strike of the mantis shrimp, Hemisquilla. J Exp Zool 179:395–416
Page SG (1965) A comparison of the fine structure of frog slow and twitch muscle fibers. J Cell Biol 26:477–497
Page SG, Niedergerke R (1972) Structures of physiological interest in the frog heart ventricle. J Cell Sci 11:179–203
Pasquali-Ronchetti I (1969) The organization of the sarcoplasmic reticulum and T-system in the femoral muscle of the housefly, Musca domestica. J Cell Biol 40:269–273
Pasquali-Ronchetti I (1970) The ultrastructural organization of femoral muscles in Musca domestica (Diptera). Tissue & Cell 2:339–354
Peachey LD (1967) Membrane systems of crab fibers. Am Zool 7:505–513
Peachey LD, Eisenberg B (1978) Helicoids in the T-system and striations of frog skeletal muscle fibers seen by high voltage electron microscopy. Biophys J 22:145–154
Peachey LD, Franzini-Armstrong C (1983) Structure and function of membrane systems of skeletal muscle cells. In: Peachey LD, Adrian RH, Geiger SR (eds) Handbook of physiology, Section 10: Skeletal muscle. American Physiological Society, Bethesda, MD, pp 23–71
Peachey LD, Huxley AF (1964) Transverse tubules in crab muscle. J Cell Biol 23:70–71A
Rosenbluth J (1969) Sarcoplasmic reticulum of an unusually fast acting crustacean muscle. J Cell Biol 42:534–547
Scales DJ (1983) III Three dimensional electron microscopy of mammalian cardiac sarcoplasmic reticulum at 80 kV. J Ultrastruct Res 83:1–9
Selverston A (1967) Structure and function of the transverse tubular system in crustacean muscle fibers. Am Zool 7:515–525
Sherman RG, Atwood HL (1971) Structure and neuromuscular physiology of a newly discovered muscle in the walking leg of the lobster Homarus americanus. J Exp Zool 176:461–474
Sherman RG, Luff AR (1971) Structural features of the tarsal claw muscle of the spider Eurypelma marxi Simon. Can J Zool 49:1549–1556
Smith DS (1961) The organization of the flight muscle in a dragonfly, Aeshna sp. (Odonata). J Biophys Biochem Cytol 11:119–145
Smith DS (1966) The organization and function of the sarcoplasmic reticulum and T system of muscle cells. Progr Biophys Mol Biol 16:107–142
Smith DS, Aldrich HC (1971) Membrane systems in freeze-etched striated muscle. Tissue & Cell 3:261–281
Sommer JR, Johnson EA (1979) Ultrastructure of cardiac muscle. In: Burns R (ed) Handbook of physiology. The cardiovascular system. Bethesda, Md, Am Physiol Soc, Section 2, Vol I, pp 113–186
Sperelakis N (1971) Ultrastructure of the neurogenic heart of Limulus polyphemus. Z Zellforsch 116:443–463
Veratti E (1902) Richerche sulla fine struttura della fibra muscolare striata. Mem Ist Lombardo Cl Sci Mat Nat 19:87–133
Veratti E (1961) Investigation on the fine structure of the striated muscle fiber. J Biophys Biochem Cytol 10, No 4, suppl: 3–59 (paper from 1902 translated by Bruni C, Bennett HS, de Koven F, de Koven D)
Author information
Authors and Affiliations
Additional information
We wish to dedicate this paper to the late Graham Hoyle, whose lifetime of work and interest in the study of muscle from a comparative point of view has been an inspiration to us.
Rights and permissions
About this article
Cite this article
Franzini-Armstrong, C., Eastwood, A.B. & Peachey, L.D. Shape and disposition of clefts, tubules, and sarcoplasmic reticulum in long and short sarcomere fibers of crab and crayfish. Cell Tissue Res. 244, 9–19 (1986). https://doi.org/10.1007/BF00218376
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00218376