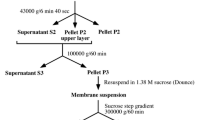
Summary
Ferritin-concanavalin A (Fer-Con A) was used to label the apical plasma membrane of the lactating cell to determine whether membrane internalization takes place. Rat glands were infused in vivo via the teat with 0.2 mg of Fer-Con A in 0.2 ml tris buffer (pH 7.0) containing 0.1% trypan blue, the latter acting as a marker of the infusate. Tissues were obtained from separate animals 5, 10 and 60 min postinfusion. Fer-Con A was seen in alveolar lumina bound to the outer surfaces of apical plasma membrane, microvilli and milk fat globules. It was observed within lactating cells on the inner membrane surfaces of endocytotic vesicles, Golgi cisternae, and secretory vesicles containing casein micelles, and in multivesicular bodies and lysosomes. Internalization of the ferritin-lectin conjugate into casein-containing secretory vesicles was detectable in the 5-min postinfusion tissue. Lysosomes were the only structures in control tissue that contained particles bearing some resemblance to Fer-Con A. The data provide evidence that apical plasma membrane is internalized and distributed to a number of intracellular compartments.
Similar content being viewed by others
References
Ainsworth SK, Karnovsky MJ (1972) An ultrastructural staining method for enhancing the size and electron opacity of ferritin in thin sections. J Histochem Cytochem 20:225–229
Beaudoin AR, Paquet MR, Lord A, Dionne L (1978) Exocytosis in mammalian cells. II. Topography of ferritin-concanavalin A conjugate and ruthenium red binding sites on the luminal surface of pancreatic acinar cells. J Ultrastruct Res 63:170–177
Buchheim W, Welsch U (1973) Evidence for the submicellar composition of casein micelles on the basis of electron microscopical studies. Neth Milk Dairy J 27:163–180
Farquhar MG (1983) Multiple pathways of exocytosis, endocytosis, and membrane recycling: validation of a Golgi route. Fed Proc 42:2407–2413
Franke WW, Luder MR, Kartenbeck J, Zerban H, Keenan TW (1976) Involvement of vesicle coat material in casein secretion and surface regeneration. J Cell Biol 69:173–195
Herzog V, Farquhar MG (1977) Luminal membrane retrieved after exocytosis reaches most Golgi cisternae in secretory cells. Proc Natl Acad Sci USA 74:5073–5077
Heuser JE, Reese TS (1973) Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol 57:315–344
Keenan TW, Franke WW, Mather IH, Morre DJ (1978) Endomembrane composition and function in milk formation. In: Lactation Vol 4. Academic Press, New York and London, pp 405–436
Leary HL Jr, Larson BL (1982) Intracellular transport of IgG1 and IgG2 through the prepartum alveolar cell. J Dairy Sci 65, Suppl 1:82 (Abstract)
Muller WA, Steinman RM, Cohn ZA (1980) The membrane protein of the vacuolar system II. Bidirectional flow between secondary lysosomes and plasma membrane. J Cell Biol 86:304–314
Nicolson GL (1974) The interaction of lectins with animal cell surfaces. Int Rev Cytol 39:89–179
Nicolson GL, Singer SJ (1974) The distribution and asymmetry of mammalian cell surface saccharides utilizing ferritin-conjugated plant agglutinins as specific saccharide stains. J Cell Biol 60:236–248
Oliver C, Hand AR (1981) Membrane retrieval in exocrine acinar cells. In: Hand AR, Oliver C (eds) Methods in cell biology Vol 23. Academic Press, New York, pp 429–444
Pastan IH, Willingham MC (1981) Journey to the center of the cell: Role of receptosomes. Science 214:504–509
Patton S, Hubert J (1983) Binding of concanavalin A to milk fat globules and release of the lectin-membrane complex by Triton X-100. J Dairy Sci (in press)
Patton S, Keenan TW (1975) The milk fat globule membrane. Biochim Biophys Acta 415:273–309
Pearse BMF, Bretscher MS (1981) Membrane recycling by coated vesicles. Ann Rev Biochem 50:85–101
Petty HR, Ware BR (1981) The inflammatory macrophage-concanavalin A interaction: A thin-section and scanning electron microscope and Laser Doppler electrophoretic investigation of surface events. J Ultrastruct Res 75:97–111
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Roth TF, Porter KR (1964) Yolk protein uptake in the oocyte of the mosquito Aedes aegyptii L. J Cell Biol 20:313–332
Saacke RG, Heald CW (1974) Cytological aspects of milk formation and secretion. In: Lactation Vol. 2. Academic Press, New York and London, pp 148–189
Sasaki M, Keenan TW (1979) Ultrastructural characterization of carbohydrate distribution on milk lipid globule membrane. Cell Biol Int Rep 3:67–74
Sasaki M, Eigel WN, Keenan TW (1978) Lactose and major milk proteins are present in secretory vesicle-rich fractions from lactating mammary gland. Proc Natl Acad Sci USA 75:5020–5024
Silverstein SC, Steinman RM, Cohn ZA (1977) Endocytosis. Ann Rev Biochem 46:669–722
Tulkens P, Sneider YJ, Trouet A (1977) The fate of plasma membrane during endocytosis. Biochem Soc Trans 5:1809–1815
Welsch U, Singh S, Buchheim W, Patton S (1982) Evidence of plasma membrane recycling in the lactating cell by concanavalin A-ferritin labeling. J Dairy Sci 65: Suppl 1:83–84
Wooding FBP (1977) Comparative mammary fine structure. Symp Zool Soc Lond 41:1–41
Zweig S, Singer SJ (1979) Concanavalin A-induced endocytosis in rabbits reticulocytes and its decrease with reticulocyte maturation. J Cell Biol 80:487–491
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Welsch, U., Singh, S., Buchheim, W. et al. Internalization of ferritin-concanavalin A by the lactating mammary cell in vivo. Cell Tissue Res. 235, 433–438 (1984). https://doi.org/10.1007/BF00217870
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00217870