Summary
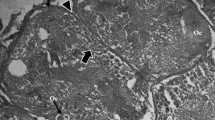
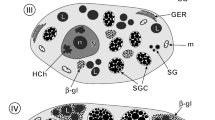
Quantitative light- and electron-microscopic autoradiography was used to evaluate metabolic processes that occur during late developmental stages (10–14) of oogenesis in Drosophila melanogaster. Major differences in radiolabelling patterns were found after in vivo (10–45 min) uptake of [3H]-monosaccharides and [3H]-L-lysine. Several different methods of data analysis were required to facilitate interpretation of these patterns. [3H]-L-lysine produced extensive cytoplasmic labelling at all developmental stages. In addition, about 15% of alpha yolk spheres were intensely labelled at stage 10, reflecting the incorporation of radiolabelled vitellogenins synthesized during the incubation period. Subsequent stages showed low silver grain density over alpha yolk spheres until stage 14, when a burst of [3H]-L-lysine incorporation by most alpha spheres was observed, possibly indicative of a maturation process for embryogenesis. [3H]-D-glucose and [3H]-D-galactose (10 min, in vivo) both induced intense labelling of the beta yolk spheres in a manner suggesting in situ assembly beginning at early stage 13. Inasmuch as the polysaccharide of beta yolk spheres has the properties of glycogen (e.g., rosette structure digested by α-amylase) and the radiolabelled monosaccharides were introduced intraabdominally, it is evident that transport systems as well as enzymes utilizing glucose and galactose for glycogenesis must be readily available. It is notable that wide-spread labelling of egg chambers was elicited by [3H]-D-glucose and [3H]-D-galactose (e.g., nurse cells, follicle cells, chorion, vitelline membrane), but the labelling induced by [3H]-N-acetylmannosamine was restricted mainly to the endochorion. A possible role of microtubules in distribution and assembly of yolk spheres was inferred when colchicine, admixed to the culture medium (2–5 ppm), produced abnormal distribution and diminution in number of both alpha and beta yolk spheres. In addition to revealing previously unknown metabolic events of vitellogenesis, the results provide additional criteria for stage characterization as well as a means to specifically label certain macromolecules for purposes of isolation.
Similar content being viewed by others
References
Bennett G, O'Shaughnessy D (1981) The site of incorporation of sialic acid residues into glycoproteins and the subsequent fates of these molecules in various rat and mouse cell types as shown by radioautography after injection of [3H]-N-acetylmannosamine. I. Observations in hepatocytes. J Cell Biol 88:1–15
Bownes M (1979) Three genes for three yolk proteins in Drosophila melanogaster. FEBS Lett 100:95–98
Bownes M (1980) The use of yolk protein variations in Drosophila species to analyze the control of vitellogenesis. Differentiation 16:109–116
Bownes M, Hames BD (1977) Accumulation and degradation of three major yolk proteins in Drosophila melanogaster. J Exp Zool 200:149–156
Carpenter ATC (1975) Electron microscopy of meiosis in Drosophila females. I. Structure, arrangement, and temporal change of the synaptonemal complex in wildtype. Chromosoma 51:157–182
Ciechanover A, Elias S, Heller H, Ferber S, Hershko A (1980) Characterization of the heat stable polypeptide of the ATP-dependent proteolytic system from reticulocytes. J Biol Chem 255:7525–7528
Coimbra A, Leblond CP (1966) Sites of glycogen synthesis in rat liver cells as shown by electron microscope radioautography after administration of glucose-H3. J Cell Biol 30:151–175
Cummings MR, King RC (1969) The cytology of the vitellogeneic stages of oogenesis in Drosophila melanogaster. I. General staging characteristics. J Morphol 128:427–442
David J (1962) A new medium for rearing Drosophila in axenic conditions. Dros Inform Serv 36:128
David J, Merle J (1968) A re-evaluation of the duration of egg chamber stages in oogenesis of Drosophila melanogaster. Dros Inform Serv 43:122–123
Dustin P (1978) Microtubules. Springer-Verlag, Berlin Heidelberg New York
Engels W, Bier K (1967) Zur Glykogenspeicherung während der Oogenese und ihrer vorzeitigen Auslösung durch Blockierung der RNS-Versorgung (Untersuchungen an Musca domestica L.). Wilhelm Roux's Arch 158:64–88
Giorgi F (1979) In vitro induced pinocytotic activity by a juvenile hormone analogue in oocytes of Drosophila melanogaster. Cell Tissue Res 203:241–247
Giorgi F, Deri P (1976) Cytochemistry of late ovarian chambers of Drosophila melanogaster. Histochem 48:325–334
Giorgi F, Jacob J (1977) Recent findings on oogenesis of Drosophila. II. Further evidence on the origin of yolk platelets. J Embryol Exp Morphol 38:125–138
Green LH, Brandis JW, Turner RF, Raff RA (1975) Cytoplasmic microtubule proteins of the embryo of Drosophila melanogaster. Biochemistry 14:4487–4491
Gutzeit HO (1980) Yolk synthesis in ovarian follicles of Drosophila. Wilhelm Roux's Arch 189:221–224
Haddad A, Smith MD, Herscovics A, Nadler NJ, Leblond CP (1971) Radioautographic study of in vivo and in vitro incorporation of fucose-3H into thyroglobulin by rat thyroid follicular cells. J Cell Biol 49:856–882
Hagedorn HH, Kunkel JG (1979) Vitellogenesis and vitellin in insects. Ann Rev Entomol 24:475–505
Harms E, Reutter W (1974) Half-life of N-acetylneuraminic acid in plasma membranes of rat liver and Morris hepatoma 7777. Cancer Res 34:3165–3172
Johnson CC, King RC (1974) Oogenesis in the ocelliless mutant of Drosophila melanogaster Meigen (Diptera: Drosophilidae). Int J Insect Morphol Embryol 3:385–395
King RC (1960) Oogenesis in adult Drosophila melanogaster. IX. Studies on the cytochemistry and ultrastructure of developing oocytes. Growth 24:265–323
King RC (1970) Ovarian development in Drosophila melanogaster. Academic Press, New York
King RC, Koch EA (1963) Studies of ovarian follicle cells of Drosophila. Q J Microsc Sci 104:297–320
King RC, Rubinson AC, Smith RF (1956) Oogenesis in adult Drosophila melanogaster. Growth 20:121–157
King RC, Bentley RM, Aggarwal SK (1966) Some of the properties of the components of Drosophila ooplasm. Am Nat 100:365–367
Koch EA, King RC (1966) The origin and early differentiation of the egg chamber of Drosophila melanogaster. J Morphol 119:283–304
Koch EA, Spitzer RH (1978) Oogenesis in Drosophila melanogaster: radioincorporation and PCF- binding in vivo and molecular weights of water soluble proteins. XIV International Congress of Genetics (Moscow), Part I, p 588
Koch EA, Spitzer RH (1979) Events in oogenesis in Drosophila melanogaster as revealed by in vivo uptake of [3H]-D-galactose. Am Zool 19:999
Koch EA, Spitzer RH (1981) Oogenesis in Drosophila melanogaster: assembly of beta yolk spheres. Genetics 97:s58
Koch EA, Smith PA, King RC (1967) The division and differentiation of Drosophila cystocytes. J Morphol 121:55–70
Kopriwa BM (1973) A reliable standardized method for ultrastructural electron microscopic radioautography. Histochemie 37:1–17
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680–685
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Loyd JE, Raff EC, Raff RA (1981) Site and timing of synthesis of tubulin and other proteins during oogenesis in Drosophila melanogaster. Dev Biol 86:272–284
Mahowald AP (1972a) Oogenesis. In: Counce SJ, Waddington CH (eds) Developmental systems: Insects, Vol 1. Academic Press, London, pp 1–47
Mahowald AP (1972b) Ultrastructural observations on oogenesis in Drosophila. J Morphol 133:29–48
Mahowald AP, Kambysellis MP (1980) Oogenesis. In: Ashburner M, Wright TRF (eds) The genetics and biology of Drosophila, Vol 2d. Academic Press, London, pp 141–224
Mahowald AP, Strassheim JM (1970) Intercellular migration of centrioles in the germarium of Drosophila melanogaster. An electron microscopic study. J Cell Biol 45:306–320
Margaritis LH, Kafatos FC, Petri WH (1980) The eggshell of Drosophila melanogaster. I. Fine structure of the layers and regions of the wild-type eggshell. J Cell Sci 43:1–35
Petri WH, Wyman AK, Kafatos FC (1976) Specific protein synthesis in cellular differentiation. III. The eggshell proteins of Drosophila melanogaster and their program of synthesis. Dev Biol 49:185–199
Postlethwait JH, Kaschnitz R (1978) The synthesis of Drosophila melanogaster vitellogenins in vivo, in culture and in a cell-free translation system. FEBS Lett 95:247–251
Postlethwait JH, Shirk PD (1981) Genetic and endocrine regulation of vitellogenesis in Drosophila. Am Zool 21:687–700
Quattropani SL, Anderson E (1969) The origin and structure of the secondary coat of the egg of Drosophila melanogaster. Z Zellforsch 95:495–510
Rösner H, Wiegandt H, Rahmann H (1973) Sialic acid incorporation into gangliosides and glycoproteins of the fish brain. J Neurochem 21:655–665
Spralding AC, Mahowald AP (1979) Identification and genetic localization of mRNAs from ovarian follicle cells of Drosophila melanogaster. Cell 16:589–598
Spralding AC, Mahowald AP (1980) Amplification of genes for chorion proteins during oogenesis in Drosophila melanogaster. Proc Natl Acad Sci USA 77:1096–1100
Srdić Z, Reinhardt C, Beck H, Gloor H (1979) Autonomous yolk protein synthesis in ovaries of Drosophila cultured in vivo. Wilhelm Roux's Archiv 187:255–266
Telfer WH (1975) Development and physiology in the oocyte-nurse cell syncytium. Adv Insect Physiol 11:223–319
Waring GL, Mahowald AP (1979) Identification and time of synthesis of chorion proteins in Drosophila melanogaster. Cell 16:599–607
Warren TG, Mahowald AP (1979) Isolation and partial chemical characterization of the three major yolk polypeptides from Drosophila melanogaster. Dev Biol 68:130–139
Warren TG, Brennan MD, Mahowald AP (1979) Two processing steps in maturation of vitellogenin polypeptides in Drosophila melanogaster. Proc Natl Acad Sci USA 76:2848–2852
Weber K, Osborn M (1969) The reliability of molecular weight determinations by dodecyl sulfatepolyacrylamide gel electrophoresis. J Biol Chem 244:4406–4412
Wilkinson KD, Urban MK, Haas AL (1980) Ubiquitin is the ATP-dependent proteolysis factor I of rabbit reticulocytes. J Biol Chem 255:7529–7532
Whur P, Herscovics A, Leblond CP (1969) Radioautographic visualization of the incorporation of galactose-3H and mannose-3H by rat thyroids in vitro in relation to the stages of thyroglobulin synthesis. J Cell Biol 43:289–311
Author information
Authors and Affiliations
Additional information
This study was supported in part by the Asthmatic Children's Aid of Chicago, the Dr. Morris A. Kaplan Foundation and the General Research Support Grant, RR5366 of the NIH. The authors wish to thank Neila S. English, Irene Giardiana and Cynthia Uphoff-Smith for their technical assistance. The authors are also grateful to Dr. Robert C. King for his critical reading of the manuscript.
Rights and permissions
About this article
Cite this article
Koch, E.A., Spitzer, R.H. Autoradiographic studies of protein and polysaccharide synthesis during vitellogenesis in Drosophila . Cell Tissue Res. 224, 315–333 (1982). https://doi.org/10.1007/BF00216876
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216876