Summary
Previous studies have revealed a remarkable capacity of intracerebral grafts of embryonic brain tissue to establish extensive axonal connections with denervated areas in the brains of adult rats. In the present study we have explored the possibilities of using grafts in the spinal cord to substitute for the loss of noradrenergic brain-stem inputs to the severed spinal cord. Intraspinal grafts of embryonic pontine noradrenergic neurons were made into the lower thoracic region of adult rats. Three different surgical techniques were tested: (i) grafting to a small central cavity in the spinal-cord grey matter; (ii) grafting to a small subpial cavity involving removal of the dorsolateral third of the spinal-cord matter; (iii) grafting to the gap between the rostral and caudal stumps of the spinal cord after a nearly complete subpial transection. The results indicate that direct contact with the vessel-rich pia is essential for good survival of the grafts. Provided that the pia was left intact, the scarring around the grafts was minimal and the grafts fused well with both the grey and white matter of the cord. In the subpially transected cord, a brain-stem graft taken from a young embryonic donor fused well with both the rostral and the caudal stumps of the severed cord and thus restored tissue continuity across the gap.
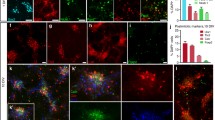
Large numbers of catecholamine (CA)-containing and non-monoaminergic cells were present in the transplants after 3–6 months. CA fluorescence histochemistry in combination with injections of fluorescent retrograde tracers revealed that both noradrenergic and non-monoaminergic neurons in the grafts had grown to reinnervate large segments of the host spinal cord. In those cases where the transplant had fused well with the cord, abundant CA-fluorescent axons could be traced across the graft-cord junction. They course along the grey and white matter of the host cord to reestablish a new CA terminal plexus in the grey matter as far as 12 mm from the graft.
Similar content being viewed by others
References
Bentivoglio M, Kuypers HGJM, Catsman-Berrevoets CE, Dann O (1979) Fluorescent retrograde neuronal labeling in rat by means of substances binding specifically to adeninethymine rich DNA. Neurosci Lett 12:235–240
Björklund A, Skagerberg G (1979) Simultaneous use of retrograde fluorescent tracers and fluorescence histochemistry for convenient and precise mapping of monoaminergic projections and collateral arrangements in the CNS. J Neurosci Met 1:261–277
Björklund A, Stenevi U (1977) Reformation of the severed septohippocampal cholinergic pathway in the adult rat transplanted septal neurons. Cell Tissue Res 185:289–302
Björklund A, Stenevi U (1979a) Reconstruction of brain circuitries by neuronal transplants. Trends in Neurosci 2:301–306
Björklund A, Stenevi U (1979b) Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res 177:555–560
Björklund A, Segal M, Stenevi U (1979) Functional reinnervation of rat hippocampus by locus coeruleus implants. Brain Res 170:409–426
Björklund A, Dunnett SB, Stenevi U, Lewis ME, Iversen SD (1980) Reinnervation of the denervated striatum by substantia nigra transplants: functional consequences as revealed by pharmacological and sensorimotor testing. Brain Res 199:307–333
Björklund A, Stenevi U, Dunnett SB, Iversen SD (1981) Functional reactivation of the deafferented neostriatum by nigral transplants. Nature 289:497–499
Das GD, Hallas BH (1978) Transplantation of brain tissue in the brain of adult rats. Experientia 34:1304–1306
Das GD, Hallas BH, Das KG (1980) Transplantation of brain tissue in the brain of rat. I. Growth characteristics of neocortical transplants from embryos of different ages. Am J Anat 158:135–145
David S, Aguayo AJ (1981) Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214:931–933
Descarries L, Saucier G (1972) Disappearance of the locus coeruleus in the rat after intraventricular 5-hydroxy-dopamine. Brain Res 37:301–316
Dunnett SB, Björklund A, Stenevi U, Iversen SD (1981a) Behavioural recovery following transplantation of substantia nigra in rats subjected to 6-OHDA lesions of the nigrostriatal pathway. I. Unilateral lesions. Brain Res 215:147–161
Dunnett SB, Björklund A, Stenevi U, Iversen SD (1981b) Behavioural recovery following transplantation of substantia nigra in rats subjected to 6-OHDA lesions of the nigrostriatal dopamine pathway. II. Bilateral lesions. Brain Res 229:457–470
Forssberg H, Grillner S (1973) The locomotion of the acute spinal cat injected with Clonidine i.v. Brain Res 50:184–186
Freed WJ, Perlow MJ, Karoum F, Seiger A, Olson L, Hoffer BJ, Wyatt RJ (1980) Restoration of dopaminergic function of grafting of fetal rat substantia nigra to the caudate nucleus: Long-term behavioral, biochemical and histochemical studies. Ann Neurol 8:510–519
Grillner S (1973) Locomotion in the spinal cat. In: Stein RB, Pearson KG, Smith SR, Bedford JB (eds) Control of posture and locomotion. Plenum Press, New York, pp 515–535
Grillner S (1980) Brainstem control of motor pattern and locomotion. Neurosci Res Prog Bull 18:58–63
Jaeger CB, Lund RD (1980) Transplantation of embryonic occipital cortex to the brain of newborn rats. An autoradiographic study of transplant histogenesis. Exp Brain Res 40:265–272
Jankowska E, Jukes MGM, Lund S, Lundberg A (1967a) The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting effects from the flexor reflex afferents. Acta Physiol Scand 70:369–388
Jankowska E, Jukes MBM, Lund S, Lundberg A (1967b) The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurons transmitting effects from the flexor reflex afférents. Acta Physiol Scand 70:389–402
Kao CC (1981) Spinal cord reconstruction after traumatic injury. In: Windle WF (ed) The spinal cord and its reaction to traumatic injury, Vol. 18, Marcel Dekker, Inc., New York Basel, pp 271–290
Kao CC, Chang LW, Bloodworth JMBJr (1977) Axonal regeneration across transected mammalian spinal cords: An electron microscopic study of delayed microsurgical nerve grafting. Exp Neurol 54:591–615
Kromer LF, Björklund A (1980) Embryonic neural transplants provide model systems for studying development and regeneration in the mammalian CNS. In: Di Benedetta C (ed), Elsevier/North-Holland Biomedical Press, pp 409–426
Kromer LF, Björklund A, Stenevi U (1981) Innervation of embryonic hippocampal implants by regenerating axons of cholinergic septal neurons in the adult rat. Brain Res 210:153–171
Kuypers HGJM, Bentivoglio M, van der Kooy D, Catsman-Berrevoets CE (1979) Retrograde transport of bisbenzimide and propidium iodide through axons to their parent cell bodies. Neurosci Lett 12:1–7
Lorén I, Björklund A, Falck B, Lindvall O (1980) The aluminum-formaldehyde (ALFA) histofluorescence method for improved visualization of catecholamines and indolamines. I. A detailed account of the methodology for central nervous tissue using paraffin, cryostat or Vibratome sections. J Neurosci Met 2:277–300
McLoon SC, Lund RD (1980) Specific projections of retina transplanted to rat brain. Exp Brain Res 40:273–282
Møllgard K, Lundberg JJ, Beebe BK, Björklund A, Stenevi U (1978) The intracerebrally cultured “microbrain”: A new tool in developmental neurobiology. Neurosci Lett 8:295–301
Nathaniel EJH, Nathaniel DR (1973) Regeneration of dorsal root fibers into the adult rat spinal cord. Exp Neurol 40:333–350
Nygren L, Olson L (1977) A new major projection from locus coeruleus: the main source of noradrenergic nerve terminals in the ventral and dorsal columns of the spinal cord. Brain Res 132:85–93
Nygren L, Olson L, Seiger A (1977) Monoaminergic reinnervation of the transected spinal cord by homologous fetal brain grafts. Brain Res 129:227–235
Olson L, Seiger A (1972) Brain tissue transplanted to the anterior chamber of the eye: I. Fluorescence histochemistry of the immature catecholamine and 5-hydroxy-tryptamine neurons reinnervating the rat iris. Z Zellforsch 135:175–194
Perkins CS, Carlstedt T, Mizuno K, Aguayo AJ (1980) Failure of regenerating dorsal root axons to regrow into the spinal cord. Can J Neurol Sci 7:323
Perlow MJ (1980) Functional brain transplants. Peptides Suppl. 1:101–110
Perlow MJ, Freed WJ, Hoffer BM, Seiger A, Olson L, Wyatt RJ (1979) Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science 204:643–647
Rosenstein JM, Brightman MW (1978) Intact cerebral ventricle as a site for tissue transplantation. Nature 276:83–85
Rovainen CM (1976) Regeneration of Muller and Mauthner axon after spinal transection in the larval lamprey. J Comp Neurol 168:545–554
Selzer ME (1978) Mechanism of functional recovery and regeneration after spinal cord transection in larval sea lamprey. J Physiol 277:395–408
Shik ML, Orlovsky GM (1976) Neurophysiology of locomotor automatism. Physiol Rev 56:465–501
Steeves JD, Jordan LM, Lake N (1975) The close proximity of catecholamine-containing cells to the “mesencephalic locomotor region” (MLR). Brain Res 100:663–670
Stein PSG (1978) Motor systems, with specific reference to the control of locomotion. Ann Rev Neurosci 1:61–81
Stenevi U, Björklund A, Svendgaard N-Na (1976) Transplantation of central and peripheral monoamine neurons to the adult rat brain: techniques and conditions for survival. Brain Res 114:1–20
Stenevi U, Björklund A, Dunnett SB (1980) Functional reinnervation of the denervated neostriatum by nigral transplants. Peptides 1:111–116
Stensaas LJ, Brugess PR, Horch KW (1979) Regenerating dorsal root axons blocked by spinal cord astrocytes. Neuroscience Abstracts 5:684
Wiklund L, Björklund A (1975) Abortive regeneration of catecholamine neurons after intraspinal 6-hydroxydopamine treatment in the adult rat. In: Jonsson G, Malmfors T, Sachs C (eds) Chemical tools in catecholamine research, vol. 1, North-Holland Publishing Company, pp 133–139
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nornes, H., Björklund, A. & Stenevi, U. Reinnervation of the denervated adult spinal cord of rats by intraspinal transplants of embryonic brain stem neurons. Cell Tissue Res. 230, 15–35 (1983). https://doi.org/10.1007/BF00216024
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216024