Summary
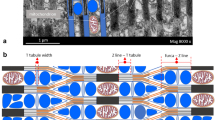
Ultrastructural parameters of muscle fibre types of the carp (Cyprinus carpio L.) were measured and compared with their contractile properties. In red fibres, which are slower than pink fibres, the relative length of the junction between the T system and the sarcoplasmic reticulum (T-SR junction) is smaller and the Z lines are thicker than in pink fibres. Pink fibres have a smaller relative length of T-SR junction than white fibres from the axial muscles. The two types of red fibres present in carp muscle also differ in their relative lengths of T-SR junction. Significant differences in the relative areas of the SR were not found.
The relative volume of myofibrils in red fibres is two-thirds that in pink fibres, a difference that is not reflected in the maximal isometric tetanic tensions of these types. Red fibres, which are less easily fatigued than pink fibres, have larger relative volumes of subsarcolemmal and intermyofibrillar mitochondria. Small pink fibres have a larger relative volume of subsarcolemmal mitochondria than large pink fibres, but have a similar relative volume of intermyofibrillar mitochondria. Small and large pink fibres differ in the relative volumes of their membrane systems, but have similar relative lengths of T-SR junction.
Similar content being viewed by others
References
Akster HA (1981) Ultrastructure of muscle fibres in head and axial muscles of the perch (Perca fluviatilis L.). A quantitative study. Cell Tissue Res 219:111–131
Akster HA (1983) A comparative study of fibre type characteristics and terminal innervation in head and axial muscle of the carp (Cyprinus carpio L.): A histochemical and electron-microscopical study. Neth J Zool 33:164–188
Akster HA, Osse JWM (1978) Muscle fibre types in head muscles of the perch Perca fluviatilis (L), Teleostei. A histochemical and electromyographical study. Neth J Zool 28:94–110
Akster HA, Granzier HLM, ter Keurs HEDJ (1985) A comparison of quantitative ultrastructural and contractile characteristics of muscle fibre types of the perch, Perca fluviatilis. L. J Comp Physiol (in press)
Barets A (1961) Contribution a l'étude des systèmes moteurs “lent” et “rapide” du muscle latéral des téléostéens. Arch Anat Morphol Exp 50 (Suppl):91–187
Bone Q (1978) Locomotor muscle. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic Press, New York, vol VII:361–424
Briggs FN, Poland JL, Solaro RJ (1977) Relative capabilities of sarcoplasmic reticulum in fast and slow mammalian skeletal muscles. J Physiol 266:587–594
Celio MR, Heizmann CW (1982) Calcium-binding protein parvalbumin is associated with fast contracting muscle fibres. Nature 297:504–506
Cullen MJ, Hollingworth S, Marshall MW (1984) A comparative study of the transverse tubular system of the rat extensor digitorum longus and soleus muscles. J Anat 138:297–308
Egginton S, Johnston IA (1982) A morphometric analysis of regional differences in myotomal muscle ultrastructure in the juvenile eel (Anguilla anguilla L). Cell Tissue Res 222:579–596
Eisenberg BR, Eisenberg RS (1982) The T-SR junction in contracting single skeletal muscle fibers. J Gen Physiol 79:1–19
Eisenberg BR, Kuda AM (1975) Stereological analysis of mammalian skeletal muscle. II. White vastus muscle of the adult Guinea Pig. J Ultrastruct Res 51:176–187
Eisenberg BR, Kuda AM (1976) Discrimination between fiber populations in mammalian skeletal muscle by using ultrastructural parameters. J Ultrastruct Res 54:76–88
Eisenberg BR, Salmons S (1981) The reorganisation of subcellular structure in muscle undergoing fast-to-slow type transformation. A Stereological study. Cell Tissue Res 220:449–471
Eisenberg BR, Kuda AM, Peter JB (1974) Stereological analysis of mammalian skeletal muscle. I. Soleus muscle of the adult guinea pig. J Cell Biol 60:732–754
Fawcett DW, Revel JP (1961) The sarcoplasmic reticulum of a fast-acting fish muscle. J Biophys Biochem Cytol 10, Suppl 4:89–104
Flitney FW (1971) The volume of the T-system and its association with the sarcoplasmic reticulum in slow muscle fibres of the frog. J Physiol 217:243–257
Focant B, Huriaux F, Johnston IA (1976) Subunit composition of fish myofibrils: the light chains of myosin. Int J Biochem 7:129–133
Focant B, Jacob MF, Huriaux F (1981) Electrophoretic comparison of the proteins of some perch (Perca fluviatilis L) head muscles. J Musc Res Cell Motil 2:295–305
Focant B, Huriaux F, Vandewalle P (1983) Electrophoretic differentiation of the fibre types of the adductor mandibulae, sternohyoideus and protractor hyoideus muscles of the carp (Cyprinus carpio L). Comp Biochem Physiol 76B:283–289
Franzini-Armstrong C (1970) Studies of the triad. I. Structure of the junction in frog twitch fibers. J Cell Biol 47:488–499
Franzini-Armstrong C (1973) Membranous systems in muscle fibers. In: Bourne GH (ed) The structure and function of muscle. Academic Press, New York, London, vol II:532–620
Gauthier GF (1979) Ultrastructural identification of muscle fiber types by immunocytochemistry. J Cell Biol 82:391–400
Gerday C, Gillis JM (1976) The possible role of parvalbumins in the control of contraction. J Physiol 258:96P
Gillis JM, Thomason D, Lefèvre J, Kretsinger RH (1982) Parvalbumins and muscle relaxation: a computer simulation study. J Musc Res Cell Motil 3:377–398
Granzier HLM, Wiersma J, Osse JWM, Akster HA, van Dommelen WMCM (1982) Contractile properties of a white- and a red-fibre type of the m. hyohyoideus of the carp (Cyprinus carpio L). J Musc Res Cell Motil 3:122
Granzier HLM, Wiersma J, Akster HA, Osse JWM (1983) Contractile properties of a white- and a red-fibre type of the m. hyohyoideus of the carp (Cyprinus carpio L). J Comp Physiol 149:441–449
Green HJ, Klug GA, Reichmann H, Seedorf U, Wiehrer W, Pette D (1984) Exercise-induced fibre type transitions with regard to myosin, parvalbumin and sarcoplasmic reticulum in muscles of the rat. Pflügers Arch 400:432–438
Hagiwara S, Takahashi K (1967) Resting and spike potentials of skeletal muscle fibres of salt-water elasmobranch and teleost fish. J Physiol 190:499–518
Hamoir G, Focant B, Distèche M (1972) Proteinic criteria of differentiation of white, cardiac and various red muscles in carp. Comp Biochem Physiol 41B:665–674
Hamoir G, Gerardin-Otthiers N, Focant B (1980) Protein differentiation of the superfast swimbladder muscle of the toadfish Opsanus tau. J Mol Biol 143:155–160
Hamoir G, Gerardin-Otthiers N, Grodent V, Vandewalle P (1981) Sarcoplasmic differentiation of head muscles of the carp Cyprinus carpio (Pisces, Cypriniformes). Mol Physiol 1:45–58
Heilmann C, Pette D (1979) Molecular transformations in sarcoplasmic reticulum of fast-twitch muscle by electro-stimulation. Eur J Biochem 93:437–446
Heilmann C, Brdiczka D, Nickel E, Pette D (1977) ATPase activities, Ca2+ transport and phosphoprotein formation in sarcoplasmic reticulum subfractions of fast and slow rabbit muscles. Eur J Biochem 81:211–222
Heizmann CW (1984) Parvalbumin, an intracellular calcium-binding protein; distribution, properties and possible roles in mammalian cells. Exp 40:910–920
Hess A (1970) Vertebrate slow muscle fibres. Physiol Rev 50:40–62
Huriaux F, Lefebvre F, Focant B (1983) Myosin polymorphism in muscles of the toadfish, Opsanus tau. J Musc Res Cell Motil 4:223–232
Johnston IA (1982) Biochemistry of myosins and contractile properties of fish skeletal muscle. Mol Physiol 2:15–29
Johnston IA, Davison W, Goldspink G (1977) Energy metabolism of carp swimming muscles. J Comp Physiol B 114:203–216
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137a-138a
Kilarski W, Kozłowska M (1983) Ultrastructural characteristic of the teleostean muscle fibers and their nerve endings. The stickleback (Gasterosteus aculeatus L). Z mikrosk-anat Forsch Leipzig 97:1022–1036
Kim DH, Witzmann FA, Fitts RH (1981) A comparison of sarcoplasmic reticulum function in fast and slow skeletal muscle using crude homogenate and isolated vesicles. Life Sci 28:2223–2229
Klug G, Reichmann H, Pette D (1983a) Rapid reduction in parvalbumin concentration during chronic stimulation of rabbit fast twitch muscle. FEBS Lett 152:180–182
Klug G, Wiehrer W, Reichmann H, Leberer E, Pette D (1983b) Relationships between early alterations in parvalbumins, sarcoplasmic reticulum and metabolic enzymes in chronically stimulated fast twitch muscle. Pflügers Arch 399:280–284
Kronnie G te, Pool CW, Scholten G, Van Raamsdonk W (1980) Myofibrillar differences among mammalian skeletal muscle fibres at the ultrastructural level. A comparison of immunocytochemical and morphological parameters. Eur J Cell Biol 22:780–788
Kronnie G te, Tatarczuch L, van Raamsdonk W, Kilarski W (1983) Muscle fibre types in the myotome of stickleback, Gasterosteus aculeatus L; a histochemical, immunohistochemical and ultrastructural study. J Fish Biol 22:303–316
Kryvi H (1977) Ultrastructure of the different fibre types in axial muscles of the sharks Etmopterus spinax and Galeus melastomus. Cell Tissue Res 184:287–300
Kubišta V, Kubštová J, Pette D (1971) Thyroid hormone induced changes in the enzyme activity pattern of energy-supplying metabolism of fast (white), slow (red), and heart muscle of the rat. Eur J Biochem 18:553–560
Mathias RT, Levis RA, Eisenberg RS (1980) Electrical models of excitation-contraction coupling and charge movement in skeletal muscle. J Gen Physiol 76:1–31
McArdle HJ, Johnston IA (1981) Ca2+-uptake by tissue sections and biochemical characteristics of sarcoplasmic reticulum isolated from fish fast and slow muscles. Eur J Cell Biol 25:103–107
Miledi R, Parker I, Zhu PH (1983) Calcium transients studied under voltage-clamp control in frog twitch muscle fibres. J Physiol 340:649–680
Miledi R, Parker I, Zhu PH (1984) Extracellular ions and excitation-contraction coupling in frog twitch muscle fibres. J Physiol 351:687–710
Müller W (1976) Subsarcolemmal mitochondria and capillarization of soleus muscle fibers in young rats subjected to an endurance training. A morphometric study of semithin sections. Cell Tissue Res 174:367–389
Nag AC (1972) Ultrastructure and adenosine triphosphatase activity of red and white muscle fibers of the caudal region of a fish, Salmo gairdneri. J Cell Biol 55:42–57
Nakajima Y (1669) Fine structure of red and white muscle fibres and their neuromuscular junctions in the snake fish (Ophio cephalus argus). Tissue and Cell 1:229–246
Page SG (1965) A comparison of the fine structures of frog slow and twitch muscle fibres. J Cell Biol 26:477–497
Page SG (1968) Fine structure of tortoise skeletal muscle. J Physiol 197:709–715
Page SG (1979) Structure and some contractile properties of fast and slow muscles of the chicken. J Physiol 205:131–145
Page SG, Huxley HE (1963) Filament length in striated muscle. J Cell Biol 19:369–390
Patterson S, Goldspink G (1972) The fine structure of red and white myotomal muscle fibres of the coalfish (Gadus virens). Z Zellforsch 133:463–474
Pool CW, van Raamsdonk W, Diegenbach PC, Mijzen P, Schenkkan EJ, van der Stell A (1976) Muscle fibre typing with sera against myosin and actin. A comparison between enzyme- and immunohistochemical classification. Acta Histochem 57:20–33
Salmons S, Gale DR, Sréter FA (1978) Ultrastructural aspects of the transformation of muscle fibre type by long term stimulation: changes in Z discs and mitochondria. J Anat 127:17–31
Schneider MF, Chandler WK (1973) Voltage dependent charge movement in skeletal muscle: a possible step in excitation-contraction coupling. Nature 242:244–246
Sitte H (1967) Morphometrische Untersuchungen an Zellen. In: Weibel ER, Elias H (eds) Quantitative methods in morphology. Springer, New York, pp 167–197
Slinde E, Kryvi H (1980) Studies on the nature of the Z-discs in skeletal muscle fibres of sharks Etmopterus spinax L and Galeus melastomus Rafinesque-Schmaltz. J Fish Biol 16:299–308
Sokal RR, Rohlf FJ (1969) Biometry. WH Freeman and Company, San Francisco
Somlyo AV (1979) Bridging structures spanning the junctional gap at the triad of skeletal muscle. J Cell Biol 80:743–750
Sréter FA (1969) Temperature, pH and seasonal dependence of Ca-uptake and ATPase activity of white and red muscle microsomes. Arch Biochem Biophys 134:25–33
Takeuchi A (1959) Neuromuscular transmission of fish skeletal muscles investigated with intracellular microelectrodes. J Cell Comp Physiol 54:211–220
Van Raamsdonk W, te Kronnie G, Pool CW, van de Laarse W (1980) An immune histochemical and enzymic characterization of the muscle fibres in myotomal muscle of the teleost Brachydanio rerio, Hamilton-Buchanan. Acta Histochem 67:200–216
Van Raamsdonk W, van 't Veer L, Veeken K, Heyting C, Pool CW (1982) Differentiation of muscle fiber types in the teleost Brachydanio rerio, the zebrafish. Posthatching development. Anat Embryol 164:51–62
Van Winkle WB, Schwartz A (1978) Morphological and biochemical correlates of skeletal muscle contractility in the cat. I. Histochemical and electron microscopic studies. J Cell Physiol 97:99–119
Van Winkle WB, Entman ML, Bornet EP, Schwartz A (1978) Morphological and biochemical correlates of skeletal muscle contractility in the cat. II. Physiological and biochemical studies. J Cell Physiol 97:121–135
Weibel ER, Bolender RP (1973) Stereological techniques for electron microscopic morphometry. In: Hayat MA (ed) Principles and techniques of electron microscopy. Van Nostrand Reinhold Company, New York, pp 237–296
Wiehrer W, Pette D (1983) The ratio between intrinsic 115 kDa and 30 kDa peptides as a marker of fibre type-specific sarcoplasmic reticulum in mammalian muscles. Febs Lett 158:317–320
Zawadowska B, Kilarski W (1983) Muscle fibre composition of the eye muscles of the carp (Cyprinus carpio L) defined on the base of histochemical observations. Folia Histochem Cytochem 21:115–126
Zubrzycka-Gaarn E, Korczak B, Osinska H, Sarzala MG (1982) Studies on sarcoplasmic reticulum from slow-twitch muscle. J Musc Res Cell Motil 3:191–212
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Akster, H.A. Morphometry of muscle fibre types in the carp (Cyprinus carpio L.). Cell Tissue Res. 241, 193–201 (1985). https://doi.org/10.1007/BF00214641
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00214641