Summary
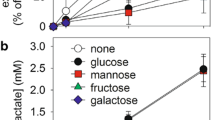
Recent reports have revealed that certain neurons do not survive in vitro in the presence of glucose, which is the primary substrate and exclusive source of energy in the brain. But these neurons can survive in the presence of low-molecular-weight agents such as pyruvate, which are supplied by glial cells (Selak et al. 1984). To test whether this result also holds true in vivo, we investigated the distribution of hexokinase, lipoic dehydrogenase, β-hydroxybutyrate dehydrogenase, and glucose-6-phosphate dehydrogenase activities in the developing rat cerebellum. Hexokinase activity was relatively higher in glial cells than in neurons. After postnatal day 8, the activity of hexokinase could hardly be detected in Purkinje cells, whereas it was highest in Bergmann glial cells. Purkinje cells were the only type of neuron with high levels of lipoic dehydrogenase at all ages tested. β-Hydroxybutylate dehydrogenase activity was also high in Purkinje cells, especially in those from young rats. Relatively high glucose-6-phosphate dehydrogenase activity was demonstrated in basket and stellate cells from adult brain. Thus, it appears that, in vivo, certain neurons utilize relatively little glucose, and it is indeed possible that glial cells may supply some substance(s) other than glucose, for example pyruvate, as the primary source of energy.
Similar content being viewed by others
References
Altman J (1972) Postnatal development of the cerebellar cortex in the rat: I The external germinal layer and the transitional moleculear layer. J Comp Neurol 145:353–398
Balogh KJ (1964) Dihydrolipoic dehydrogenase activity: A step in formation of acyl-coenzyme A, demonstrated histochemically. J Histochem Cytochem 12:404–412
Facci L, Skaper SD, Varon S (1985) Specific replacements of pyruvate for trophic support of central and peripheral nervous system neurons. J Neurochem 45:926–934
Gesink DS, Wilson JE (1985) A histochemical study of the distribution of β-hydroxybutyrate dehydrogenase in developing rat cerebellum. J Neurochem 44:1308–1311
Graham JF, Cummins CJ, Smith BH, Kornblith PL (1985) Regulation of hexokinase in cultured gliomas. Neurosurgery 17:537–542
Grossbard L, Schimke RT (1966) Multiple hexokinases of rat tissues: Purification and comparison of soluble forms. J Biol Chem 241:3546–3560
Hawkins RA, Mans AM, Davis WD, Viña ZJR, Hibbard LS (1985) Cerebral glucose use measured with [14C] glucose labeled in the 1, 2, or 6 position. Am J Physiol 248:C170-C176
Iijima K, Imai K (1976) Histochemical studies on the morphology of the Golgi apparatus and on the enzymes of carbohydrate metabolism in various nuclei of the rat mesencephalon. Histochemistry 46:209–227
Kao-Jen J, Wilson JE (1980) Localization of hexokinase in neural tissue: Electron microscopic studies of rat cerebellar cortex. J Neurochem 35:667–678
Kato T, Lowry OH (1973) Enzymes of energy-converting systems in individual mammalian nerve cell bodies. J Neurochem 20:151–163
Kaur G, Singh R, Baquer NZ (1983) Changes in hexokinase isoenzymes in regions of rat brain during insulin-induced hypoglycemia. J Neurochem 41:594–596
Lowry OH, Passonneau JV (1964) The relationships between substrates and enzymes of glycolysis in brain. J Biol Chem 239:31–42
Martinez R, Toledano A (1970) Histochemical study of the metabolism of the ketone bodies in the nervous system: IV. Localization of the D(-)-β-hydroxybutyric dehydrogenase in cerebellum and medulla oblongata. Acta Histochem 38:218–226
Meijer AEFH (1967) Histochemical method for the demonstration of the activity of hexokinase and glucokinase. Acta Histochem 28:286–290
Müller HW, Beckh S, Seifert W (1984) Neurotrophic factor for central neurons. Proc Natl Acad Sci USA 81:1248–1252
Okada Y, Yoneda K (1983) Effects of accumulation of phosphocreatine on the survival time of thin hippocampal slices from the guinea pig during deprivation of both oxygen and glucose. Neurosci Lett 41:119–124
Rudolph G, Klein HJ (1964) Histochemische Darstellung und Verteilung der Glukose-6-Phosphat-Dehydrogenase in normalen Rattenorganen. Histochemie 4:238–251
Selak I, Skaper SD, Varon S (1985) Pyruvate participation in the low molecular weight trophic activity for central nervous system neurons in glia-conditioned media. J Neurosci 5:23–28
Skaper SD, Selak I, Manthorpe M, Varon S (1984) Chemically defined requirements for the survival of cultured 8-day chick embryo ciliary ganglion neurons. Brain Res 302:281–290
Simurda M, Wilson JE (1980) Localization of hexokinase in neural tissue: Immunofluorescence studies on the developing cerebellum and retina of the rat. J Neurochem 35:58–66
Sokoloff L, Fitzgerald GG, Kaufman EE (1977) Cerebral nutrition and energy metabolism. Vol 1. Nutrition and the brain. Raven Press, New York, pp 87–139
Trenkner E, Hatten ME, Sidman RL (1978) Effect of ether-soluble serum components in vitro on the behavior of immature cerebellar cells in weaver mutant mice. Neuroscience 3:1093–1100
Turek TJ, Hawkins RA, Wilson JE (1986) Correlation of hexokinase content and basal energy metabolism in discrete regions of rat brain. J Neurochem 46:983–985
Varon S, Skaper SD, Barbin G, Selak I, Manthorpe M (1984) Low molecular weight agents support survival of cultured neurons from the central nervous system. J Neurosci 4:654–658
Wilkin GP, Wilson JE (1977) Localization of hexokinase in neural tissue: Light microscopic studies with immunofluorescence and histochemical procedures. J Neurochem 29:1039–1051
Wilson JE (1983) Hexokinase. Vol 4. Handbook of Neurochemistry. Plenum Press, New York London, pp 151–172
Yoneda K, Arakawa T, Asaoka Y, Fukuoka Y, Kinugasa K, Takimoto K, Okada Y (1983) Effects of accumulation of phosphocreatine on utilization and restoration of high-energy phosphates during anoxia and recovery in thin hippocampal slices from the guinea pig. Exp Neurol 82:215–222
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Katoh-Semba, R., Keino, H. & Kashiwamata, S. A possible contribution by glial cells to neuronal energy production enzyme-histochemical studies in the developing rat cerebellum. Cell Tissue Res. 252, 133–139 (1988). https://doi.org/10.1007/BF00213834
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00213834