Summary
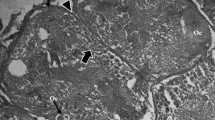
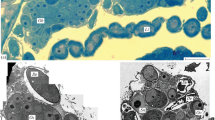
The spatial and temporal patterns of macromolecular syntheses in oocytes and somatic auxiliary cells of the snail Planorbarius corneus have been investigated by autoradiography and cytophotometry. Oogenesis has been divided into three stages, comprising early meiosis up to diplotene (stage I), previtellogenetic growth phase (stage II), and vitellogenesis (stage III). No DNA synthesis was found in any oocyte stage. In stage-I oocytes, only nucleoli were found labelled with 3H-uridine. Oocyte nuclei of stage II and III actively synthesize RNA in nucleoli and chromosomes. The most intense incorporation of uridine in chromatin probably occurs during the previtellogenesis — vitellogenesis transition period during which cytological findings suggest well developed lampbrush chromosomes. RNA synthesis in amphinucleoli of stage-III oocytes is restricted to basophilic nucleolar parts, whereas acidophilic parts (protein bodies) neither synthesize nor store RNA. During vitellogenesis oocytes incorporate amino acids into yolk platelet proteins. Radioactive proteins are found in yolk platelet precursors 5 h after injection of the tracer and in yolk platelets 3 h thereafter. The labelling pattern suggests that oocytes synthesize certain hitherto unidentified yolk components. No evidence for the participation of follicle cells in synthesis and transport of vitellogenic proteins has been obtained from autoradiography. Cytological findings suggest an important role for these cells in oogenesis. They are highly active in RNA and protein synthesis. Cellular differentiation is accompanied by polyploidization of the nuclei which attain a highest DNA content of 256 c. Polyploidization probably occurs in incremental steps as indicated by complete endomitotic chromosomal cycles. Autoradiographs show that, during vitellogenesis, oocytes do not incorporate significant amounts of glucose, and only certain follicle cells were labelled with glucose, probably indicating the synthesis of glycogen.
Similar content being viewed by others
References
Adam H, Czihak G (1964) Arbeitsmethoden der makroskopischen und mikroskopischen Anatomie. Fischer Verlag, Stuttgart
Azevedo C, Castilho F, Coimbra A (1984) Fine structure and cytochemistry of the oocyte nucleolus in the mollusc Helcion pellucidus (Prosobranchia). J Ultrastruct Res 89:1–11
Böhm N (1968) Einfluß der Fixierung und der Säurekonzentration auf die Feulgen-Hydrolyse bei 28° C. Histochemie 14:201–211
Bottke W (1973) Lampenbürstenchromosomen und Amphinukleolen in Oocytenkernen der Schnecke Bithynia tentaculata. Chromosoma (Berl) 42:175–190
Bottke W (1982) Heterochromatin in a pulmonate snail, Planorbarius corneus. Caryologia (Firenze) 35:443–448
Bottke W (1986) Immuno-localization of ferritin polypeptides in oocytes and somatic tissues of freshwater snails, Lymnaea stagnalis and Planorbarius corneus. Cell Tissue Res 243:397–404
Bottke W, Sinha I (1979) Ferritin as an exogenous yolk protein in snails. Roux's Archs Dev Biol 186:71–75
Bottke W, Sinha I, Keil I (1982) Coated-vesicle-mediated transport and deposition of vitellogenic ferritin in the rapid growth phase of snail oocytes. J Cell Sci 53:173–191
Cotelli F, Andronico F, De Santis R, Monroy A, Rosati F (1982) RNA and protein synthesis during oogenesis of Ciona intestinalis. In: Embryonic Development A, Proc IX Congr Int Soc Dev Biol Basel, AR Liss, New York, pp 381–387
Dohmen MR (1983) Gametogenesis. In: Verdonk NH, van den Biggelaar JAM, Tompa AS (eds) The Mollusca, Academic Press, London, pp 1–48
Favard P, Carasso N (1958) Origine et ultrastructure des plaquettes vitellines de la Planorbe. Arch Anat Microsc Morphol Exp 47:211–229
Fischer A, Dhainaut A (1985) The origin of yolk in the oocytes of Nereis virens (Annelida, Polychaeta). Cell Tissue Res 240:67–76
Geraerts WPM, Joosse J (1975) Control of vitellogenesis and of female accessory sex organs by the dorsal body hormone (DBH) in the hermaphroditic freshwater snail Lymnaea stagnalis. Gen Comp Endocrinol 27:450–464
Huebner E, Anderson E (1976) Comparative spiralian oogenesisstructural aspects: an overview. Am Zool 16:315–343
Jong-Brink M de, Wit A de, Kraal G, Boer HH (1976) A light and electron microscope study of oogenesis in the freshwater pulmonate snail Biomphalaria glabrata. Cell Tissue Res 171:195–219
Jong-Brink M de, Boer HH, Joosse J (1983) Mollusca. In: Adiyodi KG, Adiyodi RG (eds) Reproductive Biology of Invertebrates, Vol I. Oogenesis, oviposition, and oosorption. John Wiley and Sons Ltd, New York, pp 297–355
Jong-Brink M de, Bergamin-Sassen MJ, Kuyt JRM, Tewari-Kanhai AL (1986) Enzyme cytochemical evidence for the activation of adenylate cyclase in the follicle cells of vitellogenic oocytes by the dorsal body hormone in the snail Lymnaea stagnalis. Gen Comp Endocrinol 63:212–226
Lundelius JW, Freeman G (1986) A photoperiod gene regulates vitellogenesis in Lymnaea peregra (Mollusca: Gastropoda: Pulmonata). Int J Invert Reprod Dev 10:201–226
McCann-Collier M (1979) RNA synthesis during Ilyanassa oogenesis: an autoradiographic study. Dev Growth Differ 21:391–399
Medina A, Garcia JC, Moreno FJ, Lopez-Campos JL (1986) Comparative studies on the histology of the ovotestis in Hypselodoris tricolor and Godiva banyulensis (Gastropoda, Opisthobranchia), with special reference to yolk formation. J Morphol 188:105–118
Miksys SL, Saleuddin ASM (1987) Ferritin in mantle pore cells and its role in reproduction of Helisoma duryi (Mollusca: Pulmonata). J Exp Zool 242:75–83
Ruthmann A (1966) Methoden der Zellforschung. Franckh 'sche Verlagshandlung, Stuttgart
Selman K, Wallace RA (1978) An autoradiographic study of vitellogenesis in the squid, Loligo pealei. Tissue Cell 10:599–608
Ward RT, Opresko L, Wallace RA (1985) A comparison of the proteins of yolk platelets and intramitochondrial crystals in the oocytes of the bullfrog. Dev Biol 112:59–65
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bottke, W., Tiedtke, A. An autoradiographic and cytophotometric study of oogenesis in a pulmonate snail, Planorbarius corneus . Cell Tissue Res. 252, 67–77 (1988). https://doi.org/10.1007/BF00213827
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00213827