Summary
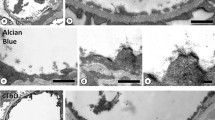
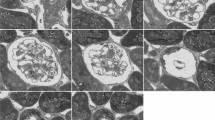
A new endothelial cell structure, named the endothelial pocket, has been found by combined transmission and scanning electron microscopic studies of renal peritubular capillaries. Transmission EM observations made on these and other fenestrated capillaries demonstrated that each pocket consists of an attenuated fold of fenestrated endothelium that projects 200 nm into the lumen above the rest of the endothelial surface. Beneath this luminal fold, there is a space and then another layer of fenestrated endothelium which abuts the basal lamina. The linear density of endothelial pockets was measured in the capillaries of the kidney cortex, intestinal mucosa and exocrine pancreas in mice and determined to be 0.067, 0.017 and 0.007 pockets·μm-1 respectively. Cationic ferritin decoration of the anionic sites on the luminal surface of the endothelium in these capillary beds revealed that both unlabelled and labelled diaphragms are clustered. In such specimens, the majority of the luminal diaphragms on endothelial pockets did not have cationic ferritin binding sites detectable by either scanning or transmission EM. On this account as well as on account of their general morphology, endothelial pockets appear to be multifold versions of the simple transendothelial channel.
Similar content being viewed by others
References
Bearer E, Orci L (1985) Endothelial fenestral diaphragms. A quick freeze, deep etch study. J Cell Biol 100:418–428
Bennett HS, Luft JH, Hampton JC (1959) Morphological classification of vertebrate blood capillaries. Am J Physiol 196:381–390
Clementi F, Palade GE (1969) Intestinal capillaries I. Permeability to peroxidase and ferritin. J Cell Biol 41:33–58
Elfvin L (1965) The ultrastructure of capillary fenestrae on the adrenal medulla of the rat. J Ultrastruct Res 12:687–704
Farquhar MG (1961) Fine structure and function of capillaries in the anterior pituitary gland. Angiology 12:270–292
Friederici HH (1968) The tridimentional ultrastructure of fenestrated capillaries. J Ultrastruct Res 23:444–456
Ghinea N, Simionescu N (1985) Anionized and cationized hemeundecapeptides as probes for cell surface charge and permeability studies: differential labeling of endothelial plasmalemmal vesicles. J Cell Biol 100:606–612
Milici AJ, L'Hernault N, Palade GE (1985) Surface densities of diaphragmed fenestrae and transendothelial channels in different murine capillary beds. Circ Res 56:709–717
Peters K-R (1980a) Improved handling of structural fragile cell biological specimens during electron microscope preparations by the exchange method. J Microsc 118:429–441
Peters K-R (1980b) Penning sputtering of ultrathin films for high resolution electron microscopy. Scan Electron Microsc 1980 1:143–154
Peters K-R (1984) Generation, collection and properties of an SE-I enriched signal suitable for high resolution SEM on bulk specimens. In: Kayser DF, Niedrig H, Newburry DE, Shimizu R (eds) Electron beam interactions with solids for microscopy, microanalysis and microlithography. Scan Electron Microsc Inc, AMF O'Hare, IL 60666:363–372
Peters K-R (1986) Metal deposition by high-energy sputtering for high mangification electron microscopy. In: Koehler JK (ed) Biological electron microscopy III. Springer, New York 101–105
Peters K-R, Green SA (1983) Macromolecular structures of biological specimens are not obscured by controlled osmium impregnation. In: Bailey GW (ed) Forty-First Annual Meeting Electron Microscopic Society of America. San Francisco Press, Inc, San Francisco, CA 94101–6800:606–607
Rhodin J (1962) The diaphragm of capillary endothelial fenestrations. J Ultrastruct Res 6:171–185
Simionescu M, Simionescu N, Palade GE (1974) Morphometric data on the endothelium of blood capillaries. J Cell Biol 60:128–152
Simionescu M, Simionescu N, Palade GE (1975) Permeability of muscle capillaries to small hemepeptides. Evidence for the existence of patent transendothelial channels. J Cell Biol 64:586–607
Simionescu M, Simionescu N, Silbert J, Palade GE (1981b) Differential microdomains on the luminal surface of capillary endothelium: II. Partial characterization of their anionic sites. J Cell Biol 90:614–621
Simionescu N, Simionescu M, Palade GE (1972) Permeability of intestinal capillaries. Pathways followed by dextrans and glycogens. J Cell Biol 53:365–392
Simionescu N, Simionescu M, Palade GE (1981a) Differentiated microdomains on the luminal surface of capillary endothelium: I. Preferential distribution of anionic sites. J Cell Biol 90:605–613
Venkatachalam M, Karnovsky M (1972) Extravascular protein in the kidney. An ultrastructural study of its relation to renal peritubular capillary permeability using protein tracers. Lab Invest 27:435–444
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Milici, A.J., Peters, K.R. & Palade, G.E. The endothelial pocket. Cell Tissue Res. 244, 493–499 (1986). https://doi.org/10.1007/BF00212526
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00212526