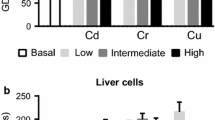
Abstract
The interactions in vitro of heavy metals Cd(II), Co(II), Cu(II), Ni(II), Pb(II), and Zn(II) with cytochrome P4501A (CYP1A) induction response and enzyme activity were studied in fish hepatoma cells PLHC-1. Cells were simultaneously exposed to heavy metals and to 3-methylcholanthrene (3-MC), an inducer of CYP1A. Heavy metals were added to the cells in different concentrations. Cytotoxicity were measured in the neutral red (NR) assay, relative CYP1A protein contents in an enzyme-linked immunosorbent assay (ELISA), and CYP1A activities in the ethoxyresorufin-O-deethylase (EROD) assay. All metals had a more pronounced effect on EROD activity than on CYP1A protein content and cytotoxicity. For the most active metal Cd(II), a 50% inhibition of EROD activity was observed at significantly lower concentrations (2.2 · 10−5 M) than a 50% reduction of CYP1A protein (5.3 · 10−5 M), and a 50% cytotoxicity (1.4 · 10−4 M). The inhibitory potency of the metals had the following order: Cd(II)>Ni(II)>Cu(II)>Co(II)=Zn(II)>Pb(II). In a second set of experiments, lysates of 3-MC—induced cells were exposed to heavy metals. Cd(II) and Cu(II) caused a 50% inhibition of EROD activity at significantly lower concentrations than in the experiments with living cells, at 8.2 · 10−6 M and 1.3 · 10−5 M, respectively, whereas the effect by Co(II) occurred at a significantly higher concentration (8.2 · 10−4 M). The results indicate that Cd(II) and Cu(II) in particular may affect the CYP1A system of the liver of fish at low concentrations through direct inhibition of the CYP1A enzyme activity. CYP1A induction response in fish liver is increasingly being used in biomonitoring programs. In the environment, interactions of CYP1A-inducing and CYP1A-inhibiting components (such as heavy metals) can be expected and must be taken into consideration.
Similar content being viewed by others
References
Alvares AP, Leigh S, Cohn J, Kappas A (1972) Lead and methyl mercury: effects of acute exposure on cytochrome P-450 and the mixed function oxidase system in the liver. J Exp Med 135: 1406–1409
Babich H, Shopsis C, Borenfreund E (1986) In vitro cytotoxicity testing of aquatic pollutants (cadmium, copper, zinc, nickel) using established fish cell lines. Ecotoxicol Environ Saf 11:91–99
Brüschweiler BJ, Würgler FE, Fent K (1995) Cytotoxicity in vitro of organotin compounds to fish hepatoma cells PLHC-1 (Poeciliopsis lucida). Aquat Toxicol 32:143–160
—, —, — (1996a) Inhibition of cytochrome P4501A by organotins in fish hepatoma cells PLHC-1. Environ Toxicol Chem 15:728–735
—, —, — (1996b) An ELISA assay for cytochrome P4501 in fish liver cells. Environ Toxicol Chem 15:592–596
Bucheli TD, Fent K (1995) Induction of cytochrome P450 as a biomarker for environmental contamination in aquatic ecosystems. Crit Rev Environ Sci Technol 25:201–268
De Matteis F (1978) Loss of liver cytochrome P-450 caused by chemicals. In: De Matteis F, Aldrigde WN (ed), Heme and bemeproteins, Handbook of exp pharmacol, Vol 44, Springer-Verlag, Berlin, pp 95–127
Degawa M, Arai H, Miura S, Hashimoto Y (1993a) Preferential inhibitions of hepatic P450LA2 expression and induction by lead nitrate in the rat. Carcinogenesis 14:1091–1094
Degawa M, Arai H, Kubota M, Hashimoto Y (1993b) Ionic lead, a unique metal ion as an inhibitor for cytochrome P4501A2 (CYP1A2) expression in the rat liver. Biochem Biophys Res Commun 200:1086–1092
Depledge MH, Weeks JM, Bjerregaard P (1994) Heavy metals. In: Calow P (ed), Handbook of ecotoxicology, Blackwell Scientific Publications, Oxford, pp 79–105
Fair PH (1986) Interaction of benzo(a)pyrene and cadmium on GSHS-transferase and benzo(a)pyrene hydroxylase in the black sea bass Centropristis striata. Arch Environ Contamin Toxicol 15:257–263
Fent K (1996) Ecotoxicology of organotin compounds. Crit Rev Toxicol 26:1–117
Fent K, Bucheli TD (1994) Inhibition of hepatic microsomal monooxygenase system by organotins in vitro in freshwater fish. Aquat Toxicol 24:107–126
Fent K, Stegeman JJ (1993) Effects of tributyltin in vivo on hepatic cytochrome P450 forms in marine fish. Aquat Toxicol 24:219–240
Förlin L, Haux C, Karlsson-Norrgren L, Runn P, Larsson A (1986) Biotransformation enzyme activities and histopathology in rainbow trout, Salmo gairdneri, treated with cadmium. Aquat Toxicol 8:51–64
Fukuhara M, Takabatake E (1982) In vitro action of cadmium on microsomal monooxygenase of rabbit lung. Biochem Pharmacol 31:3425–3429
George SG (1989) Cadmium effects on plaice liver xenobiotic and metal detoxication systems: dose-response. Aquat Toxicol 15:303–310
George SG, Young P (1986) The time course of effects of cadmium and 3-methylcholanthrene on activities of enzymes of xenobiotic metabolism and metallothionein levels in the plaice, Pleuronectes platessa. Comp Biochem Physiol 83C:37–44
Goering PL (1993) Lead-protein interactions as a basis for lead toxicity. Neurotoxicology 14:45–60
Hadley WM, Miya TS, Bousquet WF (1974) Cadmium inhibition of hepatic drug metabolism in the rat. Toxicol Appl Pharmacol 28:284–291
Hahn ME, Lamb TM, Schultz ME, Smolowitz RM, Stegeman JJ (1993) Cytochrome P4501A induction and inhibition by 3,3′,4,4′-tetrachlorobiphenyl in an Ah receptor-containing fish hepatoma cell line (PLHC-1). Aquat Toxicol 26:185–208
Hightower LE, Renfro JL (1988) Recent applications of fish cell culture to biomedical research. J Exp Zool 248:290–302
Lemaire-Gony S, Lemaire P (1992) Interactive effects of cadmium and benzo(a)pyrene on cellular structure and biotransformation enzymes of the liver of the European eel Anguilla anguilla. Aquat Toxicol 22:145–160
Maines MD, Kappas A (1977) Metals as regulators of heme metabolism. Science 198:1215–1221
Mance G (1987) Pollution threat of heavy metals in aquatic environments, Elsevier, London, pp 1–372
McFarlane GA, Franzin WG (1980) An examination of Cd, Cu, and Hg concentrations in livers of northern pike, Esox lucius, and white sucker, Catostomus commersoni, from five lakes near a base metal smelter at Flin Flon, Manitoba. Can J Fish Aquat Sci 37:1573–1578
Ryan JA, Hightower LE (1994) Evaluation of heavy metal ion toxicity in fish cells using a combined stress and cytotoxicity assay. Environ Toxicol Chem 13:1231–1240
Sassa S (1978) Toxic effects of lead, with particular reference to porphyrin and heme metabolism. In: DeMatteis F, Aldridge WN (ed), Heme and hemeproteins, Handbook exp pharmacol, Vol 44, Springer-Verlag, Berlin, pp 333–371
Segner H, Lenz D (1993) Cytotoxicity assays with the rainbow trout R1 cell line. Toxicol in Vitro 7:537–540
Sorensen EM (1991) Metal poisoning in fish, CRC Press, Boca Raton, FL, pp 1–374
Stegeman JJ, Hahn ME (1994) Biochemistry and molecular biology of monooxygenase: current perspectives on forms, functions, and regulation of cytochrome P450 in aquatic species. In: Malins DC, Ostrander GK (ed), Aquatic toxicology, Lewis Publishers, Boca Raton FL, pp 87–206
Tephly TR (1978) Inhibition of liver hemoprotein synthesis. In: De Matteis F, Aldrigde WN (ed), Heme and hemeproteins, Handbook exp pharmacol, Vol 44, Springer-Verlag, Berlin, pp 81–94
Tillitt DE, Giesy JP, Ankley GT (1991) Characterization of the H4IIE rat hepatoma cell bioassay as a tool for assessing toxic potency of planar halogenated hydrocarbons in environmental samples. Environ Sci Technol 25:87–92
Viarengo A (1989) Heavy metals in marine invertebrates: mechanisms of regulation and toxicity at the cellular level. Aquatic Sciences 1:295–317
Viarengo A, Nicotera P (1991) Possible role of Ca2+ in heavy metal cytotoxicity. Comp Biochem Physiol 100C:81–84
Zafarullah M, Olsson P-E, Gedamu L (1989) Endogenous and heavymetal-ion-induced metallothionein gene expression in salmonid tissues and cell lines. Gene 83:85–93
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brüschweiler, B.J., Würgler, F.E. & Fent, K. Inhibitory effects of heavy metals on cytochrome P4501A induction in permanent fish hepatoma cells. Arch. Environ. Contam. Toxicol. 31, 475–482 (1996). https://doi.org/10.1007/BF00212430
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00212430