Abstract
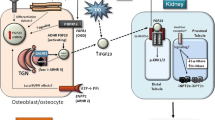
Phosphate plays a centrol role in many of the basic processes essential to the cell and organism. In particular, skeletal mineralisation is dependent on the appropriate regulation of phosphate in the body, and any disturbances in phosphate homeostasis can have severe repercussions on the integrity of bone. The kidney regulates the serum levels of phosphate by tubular mechanisms which are not fully understood. Furthermore, the processes involved in regulating renal tubular phosphate reabsorption are complex, and involve a large number of factors. It is not surprising therefore that defects in renal phosphate handling result in a failure of bone mineralisation. There are three well characterised conditions which are associated with renal tubulopathies resulting in a phosphate leak, with consequent bone disease. Two are familial, hypophosphataemic rickets (HYP), and hereditary hypophosphataemic rickets with hypercalciuria (HHRH). The third is acquired via a tumour, oncogenic hypophosphataemic osteomalacia (OHO), and may well have relevance to the inherited hypophosphataemias. Recent advances in molecular genetics are permitting the identification of genes involved in human diseases from their chromosomal location. These approaches are now being applied to the analysis of the hypophosphataemias. The isolation of the genes responsible for the renal tubulopathies will be an important achievement. Ultimately this will help to increase our understanding of the mechanisms involved in the control of phosphate handling in the body.
Similar content being viewed by others
References
Albertsen HM, Abderrahim H, Cann HM, Dausset J, Lepaslier D, Cohen D (1990) Construction and characterization of a yeast artificial chromosome library containing 7 haploid human genome equivalents. Proc Natl Acad Sci USA 87:4256–4260
Albright F, Butler AM, Bloomberg F (1937) Rickets resistant to vitamin D therapy. Am J Dis Child 54:529–547
Alitalo T, Kruse TA, Ahrens P, Albertsen HM, Eriksson AW, de la Chapelle A (1991 a). Genetic mapping of twelve marker loci in the Xp22.3-p21.2 region. Hum Genet 86:599–603
Alitalo T, Kruse TA, de la Chapelle A (1991 b) Refined localisation of the gene causing X-linked juevenile retinoschisis. Genomics 9:505–510
Aschinberg LC, Solomon LM, Zeis PM, Justice P, Rosenthal IM (1977) Vitamin D resistant rickets associated with epidermal nevus syndrome: demonstration of a phosphaturic substance in the dermal lesions. J Pediatr 91:56–60
Avner P, Amar L, Dandolo L, Guenet JL (1988) Genetic analysis of the mouse using interspecific crosses. Trends Genet 4:18–23
Barlow DP, Lehrach H (1987) Genetics by electrophoresis: the impact of pulse field gel electrophoresis on mammalian genetics. Trends Genet 3:167–171
Bell CL, Tenenhouse HS, Scriver CR (1988) Primary cultures of renal epithelial cells from X-linked hypophosphataemic (Hyp) mice express defects in phosphate transport and vitamin D metabolism. Am J Hum Genet 43:293–303
Benham F, Rowe PSN (1992) Use of Alu PCR to characterise hybrids containing multiple fragments and to generate new Xp21.3–p.22.2 markers. Genomic 12:368–376
Benham F, Hart K, Crolla J, Bobrow M, Francavilla M, Goodfellow PN (1989) A method for generating hybrids containing non-selected fragments of human chromosome. Genomics 4:509–517
Biancalana V, Briard ML, David A, Gilgenkrantz S, Kaplan J, Mathieu M, Piussan C, Poncin J, Schinzel A, Oudet C, Hanauer A (1992) Confirmation and refinement of the genetic localisation of the Coffin Lowry syndrome locus in Xp22.1–p22.2. Am J Hum Gent 50:981–987
Biancalana V, Trivier E, Weber C, Weisenbach J, Rowe PSN, O'Riordan JLH, Partington MW, Heyberger S, Oudet C, Hanauer A (1994) Construction of a high resolution linkage map for Xp22.1–p22.2 and refinement of the genetic localisation of the Coffin Lowry syndrome gene. Genomics (in press)
Bird AP (1987) CpG islands as gene markers in the vertebrate nucleus. Trends Genet 3:342–347
Burnett CH, Dent CE, Harper C, Warland BJ (1964) Vitamin D resistant rickets. Analysis of twenty four pedigrees with hereditary rickets. Analysis of twenty four pedigrees with hereditary and sporadic cases. Am J Med 36:222–232
Carey DE, Drezner MK, Hamdan JA, Mange M, Ashmad MS, Mubarak S, Nyhan WL (1986) Hypophosphataemic rickets/osteomalacia in linear sebaceous nevus syndrome: a variant of tumour induced osteomalacia. J Pediatr 109:994–1000
Chesny RW, Mazess RB, Rose P, Hamstra AJ, De Luca HF (1980 a). Supranormal 25-hydroxy vitamin D and subnormal 1,25-dihydroxy vitamin D: their role in X-linked hypophosphataemic rickets. Am J Dis Child 134:140–143
Chesny RW, Rosen JF, Hamstra AJ, De Luca HF (1980 b) Serum 1,25-dihydroxy vitamin D levels in normal children and in vitamin D disorders. Am J Dis Child 134:135–139
Christensen JF (1941) Three familial cases of atypical late rickets. Acta Paediatr Scand 28:247–270
Collins FS (1992) Positional cloning: let's not call it reverse genetics anymore. Nature Genet 1:3–6
Cowgil LD, Goldfarlb S, Lau K, Slatopolsky E, Agus ZS (1979) Evidence for an intrinsic renal tubule leak in mice with genetic hypophosphataemic rickets. J Clin Invest 63:1203–1210
Cox DR, Pritchard CA, Uglum E, Easher D, Kobori J, Myers R (1989) Segregation of the Huntington disease region of human chromosome 4 in a somatic cell hybrid. Gemonies 4:399–407
Davidai GA, Nesbitt T, Drezner MK (1990) Normal regulation of calcitriol production in Gy mice: evidence for biochemical heterogeneity in the X-linked hypophosphataemic diseases. J Clin Invest 85:334–339
Dent CE, Gertner JM (1975) Hypophosphataemic osteomalacia in fibrous dysplasia. Q J Med 45:411–420
Dent CE, Round JM, Stamp TCB (1973) Clinical aspects of metabolic bone disease. International Congress Series No. 270. Excerpta Medica, Amsterdam; pp 427–432
Devoto M, Bolini A, Enia G, Zoccali C, Romeo G (1993) A new form of X-linked rickets with hypercalciuria (HPDR-II) maps in the Xp11 region. Am J Hum Genet 53:993
Drezner MK (1990) Tumour-associated rickets and osteomalacia. In: Favus MJ (ed) Primer on metabolic bone diseases and disorders of mineral metabolism. Am Soc Bone Mineral Res, Kelseyville, Calif., pp 184–188
Drezner MK, Nesbitt T (1992) A circulating serum factor of possible hepatic origin underlies the pathogenesis of X-linked hypophosphataemic rickets (abstract). Bone Mineral 17 (Suppl 1):A521
den Dunnen JT, Bakker E, Klein-Breteler EG, Pearson PL, van Ommen JB (1987) Direct detection of more than 50% of the Duchenne muscular dystrophy mutations by field inversion gels. Nature 329:640–642
Ecarot B, Glorieux FH, Desbarats M, Travers R, Labelle L (1992 a). Defective bone formation by Hyp mouse bone cells transplanted into normal mice: evidence in favour of an intrinsic osteoblast defect. J Bone Mineral Res 7:215–220
Ecarot FH, Glorieux FH, Desbarats M, Travers R, Labelle L (1992 b). Effect of dietary phosphate deprivation and supplementation of recipient mice on bone formation by transplanted cells from normal and X-linked hypophosphataemic mice. J Bone Mineral Res 7:523–530
Ecarot-Charrier B, Glorieux FH, Travers R, Desbarats M, Bouchard F, Hinek A (1988) Defective bone formation by transplanted Hyp mouse bone cells into normal mice. Endocrinology 123:768–773
Econs MJ, Pericak-Vance MA, Betz H, Bartlett RJ, Speer MC, Drezner MK (1990) The human glycine receptor: a new probe that is linked to the X-linked hypophosphataemic rickets gene. Genomics 7:439–441
Econs MJ, Fain PR, Norman M, Speer MC, Pericak-Vance MA, Becker PA, Barker DF, Taylor A, Drezner MK (1993 a). Flanking markers define the X-linked hypophosphataemic rickets gene locus. J Bone Mineral Res 8:1149–1152
Econs MJ, Rowe PSN, Francis F, Barker DF, Speer MC, Norman M, Pericak-Vance MA, Fain PR, Weissenbach J, Read A, Becker PA, Lehrach H. O'Riordan JLH, Drezner MK (1993 b) Fine structure mapping of the human X-linked hypophos-phataemic rickets gene (abstract). J Bone Mineral Res 8 (Suppl 1):137
Econs MJ, Francis F, Rowe PSN, Speer MC, O'Riordan JLH, Lehrach H, Becker PA (1994) Dinucleotide repeat polymorphism at the DXS1683 locus. Hum Mol Genet (in press)
Eicher EM, Southard JL, Scriver CR, Glorieux FH (1976) Hypophosphataemia: mouse model for human familial hypophosphataemic (vitamin D-resistant) rickets. Proc Natl Acad Sci USA 73:4667–4671
Elles RG, Read AP, Hodgkinson KA, Watters A, Harris R (1990) Recombination or heterogeneity: is there a second locus for adult polycystic kidney disease? J Med Genet 27:413–417
Ford DM, Molitoris BA (1991) Abnormal proximal tubule apical membrane composition in X-linked hypophosphataemic mice. Am J Physiol 260:F317-F322
Francis F, Rowe PSN, Econs M, See CG, Benham F, O'Riordan JLH, Drezner MK, Hamvas RMJ, Lehrach H (1994) A YAC contig spanning the hypophosphataemic rickets disease gene candidate region. Genomics 21:229–237
Giasson SD, Brunetti MG, Danan G, Vigneault N, Carriere S (1977) Micropuncture study of renal phosphorus transport in hypophosphataemic vitamin D resistant rickets in mice. Pflugers Arch 371:33–38
Glorieux FH, Marie PJ, Pettifor J, Delvin EE (1980) Bone response to phosphate salts, ergocalciferol and calcitriol in hypophosphataemic vitamin D resistant rickets. N Engl J Med 303:1023–1031
Goss S, Harris H (1975) New method for mapping genes in human chromosomes. Nature 255:680–683
Graham JB, McFalls VW, Winters RW (1959) Familial hypophosphataemia with vitamin D resistant rickets II. Three additional kindreds of the sex linked dominant type with a genetic analysis for four such families. Am J Hum Genet 11:311–332
Greenberg BR, Winters RW, Graham JB (1960) The normal ranges of serum inorganic phosphorus and its utility as a discriminant in the diagnosis of congenital hypophosphataemia. J Clin Endocrinol Metab 20:364–379
Haddad JG, Chyu KJ, Hahn TJ, Stamp TCB (1973) Serum concentrations of 25-hydroxy vitamin D in sex linked hypophosphataemic rickets. J Lab Clin Med 81:22–27
Harvey N, Tenenhouse HS (1992) Renal Na+-phosphate-cotransport in X-linked Hyp mice responds appropriately to Na+ gradient, membrane potential, and pH. J Bone Mineral Res 7:563–571
Haussler MR, McCain TA (1977) Basic and clinical concepts related to vitamin D metabolism and action. N Engl J Med 297:974–983
Hewison M, Karmali R, O'Riordan JLH (1992) Tumour induced osteomalacia. Clin Endocrinol 37:382–384
Jones G, Vriezen D, Lohnes D, Palda V, Edwards NS (1987) Side chain oxidation of vitamin D3 and its physiological implications. Steroids 49:29–53
Kay GF, Thakker RV, Rastan S (1991) Determination of a molecular map position for Hyp using a new interspecific backcross produced by in vitro fertilisation. Genomics 11:651–657
Kinoshita Y, Fukase M, Nakade M, Fujita T (1987) Defective adaptation to a low phosphate environment by cultured renal tubular cells from X-linked hypophosphataemic (Hyp) mice. Biochem Biophys Res Commun 144:763–769
Konishi K, Nakamura M, Yamakawa H, Suzuki H, Saruta T, Hanaoka H, Davatchi T (1991) Case report: hypophosphataemic osteomalacia in von Recklinghausen neurofibromatosis. Am J Med Sci 301:322–328
Koss CH, Tihy F, Econs MJ, Murer H, Lemieux N, Tenenhouse HS (1994) Localization of a renal sodium-phosphate co-transporter gene to human chromosome 5q35. Genomics 19:176–177
Larin Z, Monaco AP, Lehrach H (1991) Yeast artificial chromosome libraries containing large inserts from mouse and human DNA. Proc Natl Acad Sci USA 88:4123–4127
Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446
Lau K, Stom MC, Goldberg M, Goldfarlb S, Gray RW, Leman J Jr, Agus ZS (1979) Evidence for a humoral phosphaturic factor in oncogenic hypophosphataemic osteomalacia (abstract). Clin Res 27:421A
Lyles KW, Berry WR, Haussler MR, Harrelson JM, Drezner MK (1980) Hypophosphataemic osteomalacia: association with prostate carcinoma. Ann Intern Med 93:275–278
Lyon MF (1988) X-chromosome inactivation and the location and expression of X-linked genes. Am J Hum Genet 42:8–16
Lyon MF, Scriver CR, Baker LRI, Tenenhouse HS, Kronick J, Mandla S (1986) The Gy mutation: another cause of X-linked hyphophosphataemia in mouse. Proc Natl Acad Sci USA 83:4899–4903
Machler M, Frey D, Gal D, Orth U, Weinker TF, Fanconi A, Schmid W (1986) X-linked dominant hypophosphataemia is closely linked to DNA markers DXS41 and DXS43 at Xp22. Hum Genet 73:271–275
Marie PJ, Travers R, Glorieux FH (1982) Healing of bone lesions with 1,25-dihydroxyvitamin D3 in the young X-linked hypophosphataemic male mouse. Endocrinology 111:904–911
Meyer RA Jr, Meyer MH (1993) Parabiosis of GYRO (Gy) mice: are there two forms of X-linked hypophosphataemia? (abstract). J Bone Mineral Res 8:135
Meyer RA Jr, Meyer MA, Gray RW (1989 a) Parabiosis suggests a humoral factor is involved in X-linked hypophosphataemia in mice. J Bone Mineral Res 4:493–500
Meyer RA Jr, Tenenhouse HS, Meyer MA, Klugerman AH (1989 b). The renal phosphate transport defect in normal mice parabiosed to X-linked hypophosphataemic mice persists after parathyroidectomy. J Bone Mineral Res 4:523–532
Miyauchi A, Fukase M, Tsutsumi M, Fujita T (1988) Hemangiopericytoma induced osteomalacia: tumour transplantation in nude mice causes hypophosphataemia and tumour extracts inhibit renal 25-hydroxyvitamin D α1-hydroxylase activity. J Clin Endocrinol Metab 67:46–53
Morgan JM, Hawley WL, Chenoweth AI, Retan WJ, Diethelm MD (1974) Renal transplantation in hypophosphataemia with vitamin-D resistant rickets. Arch Intern Med 134:549–552
Moser CR, Fessel (1974) Rheumatic manifestations of hypophos-phataemia. Arch Intern Med 134:674–678
Murphy P, Wright G, Rai GS (1985) Hypophosphataemic osteomalacia associated with prostatic carcinoma. Br Med J 290:1945
Nagawaka N, Arab N, Ghishan FK (1991) Characterisation of the defect in the Na+-phosphate transporter in vitamin D resistant hypophosphataemic mice. J Biol Chem 266:13616–13620
Nakajima S, Yamaoka K, Okada S, Pike JW, Seino Y, Haussler MR (1992) 1,25-Dihydroxyvitamin D3 does not upregulate vitamin D receptor messenger ribonucleic acid levels in hypophosphataemic mice. Bone Mineral 19:201–213
Nelson DL, Ledbetter S, Corbo L, Victoria M, Ramirez-Solis R, Webster TD, Ledbetter DH, Caskey T (1989) Alu PCR: a method for rapid isolation of human-specific sequences from complex DNA sources. Proc Natl Acad Sci USA 86:6686–6690
Nesbitt T, Coffman TM, Griffith R, Drezner Mk (1992) Cross-transplantation of kidneys in normal and Hyp mice. J Clin Invest 89:1453–1459
Nitzan DW, Horowitz AT, Darmon D, Friedlaender MM, Rubinger D, Stein P, Bab I, Popovtzer MM, Silver J (1989) Oncogenic osteomalacia: a case study. Bone Mineral 6:191–197
Ohno S (1967) Sex chromosomes and sex linked genes. Springer, Berlin
Petersen DJ, Boniface AM, Schranck FW, Rupich RC, Whyte MP (1992) X-linked hypophosphataemic rickets: a study with literature review of linear growth response to calcitriol and phopshate therapy. J Bone Mineral Res 7:583–597
Pook MA, Wrong O, Wooding C, Norden AGW, Feest TG, Thakker RV (1993) Dent's disease, a renal Fanconi syndrome with nephrocalcinosis and kidney stones, is associated with a microdeletion involving DXS255 and maps to Xp11.3. Hum Mol Genet 2:2129–2134
Popovtzer MM (1981) Tumour induced hypophosphataemic osteomalacia: evidence for a phosphaturic cyclic AMP-independent action of tumour extract (abstract). Clin Res 29:418A
Portale AA, Halloran BP, Morris RC (1987) Dietary intake of phosphate modulates the circadian rhythm in serum concentration of phosphate. J Clin Invest 80:1147–1154
Qiu ZQ, Tenenhouse HS, Scriver CR (1993) Parental origin of mutant allele does not explain absence of gene dose effect in X-linked Hyp mice. Genet Res (Camb) 62:39–43
Read AP, Thakker RV, Davies KE, Mountford RC, Brenton DP, Davies M, Glorieux FH, Harris R, Hendy GN, King A, McGlade S, Peacock CJ, Smith R, O'Riordan JLH (1986) Mapping of human X-linked hypophosphataemic rickets by multilocus linkage analysis. Hum Gent 73:267–270
Riley J, Butler R, Ogilvie D, Finniear R, Jenner D, Powell S, Anand R, Smith JC, Markham AF (1990) A novel, rapid method for the isolation of terminal sequence from yeast artificial chromosome (YAC) clones. Nucleic Acids Res 18:2887–2890
Rowe PSN, Read AP, Mountford RC, Benham F, Kruse TA, Camarino G, Davies KE, O'Riordan JLH (1992) Three DNA markers for hypophosphataemic rickets. Hum Genet 89:539–542
Rowe PSN, Goulding J, Read AP, Mountford RC, Hanauer A, Oudet C, Whyte MP, Meier-Ewert S, Lehrach H, Davies KE, O'Riordan JLH (1993 a) New markers for linkage analysis of hypophosphataemic rickets. Hum Genet 91:571–575
Rowe PSN, Goulding J, Francis F, Hanauer A, Econs M, Read A, Mandel JL, Drezner M, Lehrach H, Davies KE, O'Riordan JLH (1993 b) Isolation of cDNA clones from an oncogenic hypophosphataemic osteomalacia (OHO) tumour library by selective hybridisation using a 640 Kb YAC from a contig encompassing the HYP gene. Hum Genome Mapping 93 Proceedings, abstract 23-3
Rowe PSN, Goulding J, Read AP, Lehrach H, Francis F, Hanauer A, Oudet C, Bianacalana V, Kooh SW, Davies KE, O'Riordan JLH (1994) Refining the genetic map for the region flanking the X-linked hypophosphataemic rickets locus (Xp22.1–22.2). Hum Genet 93:291–294
Scriver CR, Reade TM, De Luca HF, Hamstra AJ (1978) Serum 1,25 dihydroxy vitamin D level in normal subjects and in patients with hereditary rickets or bone disease. N Engl J Med 299:976–979
Seshadri MS, Cornish CJ, Mason RS, Posen S (1985) Parathyroid hormone like bioactivity in tumours from patients with oncogenic osteomalacia. Clin Endocrinol 23:689–697
Taylor HC, Fallon MD, Velasco ME (1984) Oncogenic osteomalacia and inappropriate antidiuretic hormone secretion due to oat cell carcinoma. Ann Intern Med 101:786–788
Tenenhouse HS, Scriver CR, McInnes RR, Glorieux FH (1978) Renal handling of phosphate in vivo and in vitro by the X-linked hypophosphataemic male mouse: evidence for a defect in the brush border membrane. Kidney Int 14:236–244
Tenenhouse HS, Yip A, Jones G (1988) Increased renal catabolism of 1,25-dihydroxyvitamin D3 in murine X-linked hypophosphataemic rickets. J Clin Invest 81:461–465
Tenenhouse HS, Klugerman AH, Neal JL (1989) Effect of phosphonoformic acid, dietary phosphate and the Hyp mutation on kinetically distinct phosphate transport processes in mouse kidney. Biochim Biophys Acta 984:207–213
Tenenhouse HS, Werner A, Biber J, Ma S, Martel J, Roy S, Murer H (1993) Renal Na+-phosphate co-transport in X-linked Hyp mice: molecular characterisation (abstract). J Bone Mineral Res 8:139
Thakker RV, Read AP, Davies KE, Whyte MP, Weksberg R, Glorieux FH, Davies M, Mountford RC, Harris R, King A, Kim GS, Fraser D, Kooh SW, O'Riordan JLH (1987) Bridging markers defining the map position of X-linked hypophosphataemic rickets. J Med Genet 24:756–760
Thakker RV, Brockdorff N, Kay GF, Wooding C, Rastan S (1993) Molecular genetic analysis of the mouse X-linked hypophosphataemia locus by use of an interspecific backcross and linking clones (abstract). Cytogenet Cell Genet 64:189
Weidner N, Cruz DS (1987) Phosphaturic mesenchymal tumours: a polymorphous group causing osteomalacia rickets. Cancer 59:1442–1454
Werner A, Moore ML, Mantei N, Biber J, Semenza G, Murer H (1991) Cloning and expression of cDNA for Na/Pi co-transport system of kidney cortex. Proc Natl Acad Sci USA 88:9608–961
Winters RW, Graham JB, Williams TF, McFalls VW, Burnett CH (1958) A genetic study of familial hypophosphataemia and vitamin D resistant rickets with a review of the literature. Medicine 37:97–142
Wrong OM, Norden AGW, Feest TG (1990) Dent's disease a Fanconi renal tubular syndrome with hypercalciuria, tubular proteinuria, rickets, nephrocalcinosis, and eventual renal failure. Q J Med 77:1086–1087
Yamamoto T, Ecarot B, Glorieux FH (1992) Abnormal response of osteoblasts from Hyp mice to 1,25-dihydroxyvitamin D3. Bone 13:209–215
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rowe, P.S.N. Molecular biology of hypophosphataemic rickets and oncogenic osteomalacia. Hum Genet 94, 457–467 (1994). https://doi.org/10.1007/BF00211008
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00211008