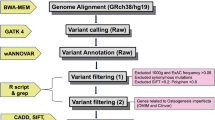
Abstract
In general, osteogenesis imperfecta (brittle bone disease) is caused by heterozygous mutations in the genes encoding the α1 or α2 chains of type I collagen (COL1A1 and COL1A2, respectively). In this study we screened these genes in a proband presenting with the severe form (type III) of osteogenesis imperfecta for mutations which might result in the phenotype. Single-strand conformation polymorphism mapping analysis was used to identify a region suspected of harbouring the mutation and subsequent sequence analysis revealed a heterozygous G to A transition in the α2(I) gene of type I collagen in the individual. The resulting substitution of the glycine at position 238 of the α chain by serine is the most N-terminal yet reported for this chain.
Similar content being viewed by others
References
Bonadio J, Holbrook KA, Gelinas RE, Jacob J, Byers PH (1985) Altered triple helical structure of type I procollagen is associated with prolonged survival in lethal perinatal osteogenesis imperfecta. J Biol Chem 260: 1734–1742
Byers PH (1990) Brittle bones — fragile molecules: disorders of collagen gene structure and expression. Trends Genet 6: 293–300
Byers PH (1993) Osteogenesis imperfecta. In: Royce PM, Steinmann B (eds) Connective tissue and its heritable disorders: molecular, genetic and medical aspects. Wiley-Liss, New York, pp 317–350
Byers PH, Wallis GA, Willing MC (1991) Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet 28: 433–442
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159
Fertala A, Westerhausen A, Morris G, Rooney JE, Prockop DJ (1993) Two cysteine substitutions in procollagen I: a glycine replacement near the N-terminus of α1(I) chain causes lethal osteogenesis imperfecta and a glycine replacement in the α2(I) chain markedly destabilizes the triple helix. Biochem J 289: 195–199
Kawasaki ES, Wang AM (1989) Detection of gene expression. In: Erlich HA (ed) PCR technology: principles and applications for DNA amplification. Stockton, New York, pp 89–97
Kuivaniemi H, Tromp G, Chu M-L, Prockop DJ (1988) Structure of a full-length cDNA clone for the preproα2(I) chain of human type I collagen. Biochem J 252: 633–640
Mackay K, Byers PH, Dalgleish R (1993a) An RT-PCR-SSCP screening strategy for detection of mutations in the gene encoding the α1 chain of type I collagen: application to four patients with osteogenesis imperfecta. Hum Mol Genet 2: 1155–1160
Mackay K, Lund AM, Raghunath M, Steinmann B, Dalgleish R (1993b) SSCP detection of a Gly565Val substitution in the proα1(I) collagen chain resulting in osteogenesis imperfecta type II. Hum Genet 91: 439–444
Mackay K, De Paepe A, Nuytinck L, Dalgleish R (1994) Substitution of glycine-172 by arginine in the α1 chain of type I collagen in a patient with osteogenesis imperfecta, type III. Hum Mutat 3: 324–326
Marini JC, Wang Q, Filie JD, Lewis MB (1993) Mutations in α2(I) collagen support a regional model of the relationship between osteogenesis imperfecta genotype and phenotype. Fifth International Conference on Osteogenesis Imperfecta, Oxford, England, 27–30 September 1993, abstract 126
Murphy G, Ward ES (1989) Sequencing of double-stranded DNA. In: Howe CJ, Ward ES (eds) Nucleic acids sequencing: a practical approach. IRL Press, Oxford, pp 99–115
Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T (1989a) Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci USA 86: 2766–2770
Orita M, Suzuki Y, Sekiya T, Hayashi K (1989b) Rapid and sensitive detection of polymorphisms using the polymerase chain reaction. Genomics 5: 874–879
Patterson E, Smiley E, Bonadio J (1989) RNA sequence analysis of a perinatal lethal osteogenesis imperfecta mutation. J Biol Chem 264: 10083–10087
Raghunath M, Mackay K, Dalgleish R, Steinmann B (1995) Genetic counselling on brittle grounds: recurring osteogenesis imperfecta due to parental mosaicism for a dominant mutation. Eur J Pediatr (in press)
Rose NJ, Mackay K, Byers PH, Dalgleish R (1993) A novel glycine to glutamic acid substitution at position 343 in the α2 chain of type I collagen in an individual with lethal osteogenesis imperfecta. Hum Mol Genet 2: 2175–2177
Rose NJ, Mackay K, Byers PH, Dalgleish R (1994a) A Gly859Ser substitution in the triple helical domain of the α2 chain of type I collagen resulting in osteogenesis imperfecta type III in two unrelated individuals. Hum Mutat 3: 391–394
Rose NJ, Mackay K, De Paepe A, Steinmann B, Punnett HH, Dalgleish R (1994b) Three unrelated individuals with perinatally lethal osteogenesis imperfecta resulting from identical Gly502Ser substututions in the α2-chain of type I collagen. Hum Genet 94: 497–503
Valli M, Mottes M, Tenni R, Sangalli A, Gomez Lira M, Rossi A, Antoniazzi F, Cetta G, Pignatti PF (1991) A de novo G to T transversion in a pro-α(I) collagen gene for a moderate case of osteogenesis imperfecta. Substitution of cysteine for glycine 178 in the triple helical domain. J Biol Chem 266: 1872–1878
Wenstrup RJ, Shrago-Howe AW, Lever LW, Phillips CL, Byers PH, Cohn DH (1991) The effects of different cysteine for glycine substitutions within α2(I) chains. Evidence of distinct structural domains within the type I collagen triple helix. J Biol Chem 266: 2590–2594
Wirtz MK, Rao VH, Glanville RW, Labhard ME, Pretorius PJ, de Vries WN, de Wet WJ, Hollister DW (1993) A cysteine for glycine substitution at position 175 in an α1(I) chain of type 1 collagen produces a clinically heterogeneous form of osteogenesis imperfecta. Connect Tissue Res 29: 1–11
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rose, N.J., Mackay, K., Byers, P.H. et al. A Gly238Ser substitution in the α2 chain of type I collagen results in osteogenesis imperfecta type III. Hum Genet 95, 215–218 (1995). https://doi.org/10.1007/BF00209405
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00209405