Abstract
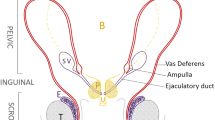
Mutations in the cystic fibrosis (CF) conductance transmembrane regulator (CFTR) gene have been detected in patients with CF and in males with infertility attributable to congenital bilateral absence of the vas deferens (CBAVD). Thirty individuals with CBAVD and 10 with congenital unilateral absence of the vas deferens (CUAVD) were analyzed by single-strand conformation analysis and denaturing gradient gel electrophoresis for mutations in most of the CFTR gene. All 40 individuals were pancreatic sufficient, but twenty patients had recurrent or sporadic respiratory infections, asthma/asthmatic bronchitis, and/or rhino-sinusitis. Agenesia or displasia of one or both seminal vesicles was detected in 30 men and other urogenital malformations were present in six subjects. Among the 40 samples, we identified 13 different CFTR mutations, two of which were previously unknown. One new mutation in exon 4 was the deletion of glutamic acid at codon 115 (ΔE115). A second new mutation was found in exon 17b, viz., an A→C substitution at position 3311, changing lysine to threonine at codon 1060 (K1060T). CFTR mutations were detected in 22 out of 30 (73.3%) CBAVD patients and in one out of 10 (10%) CUAVD individuals, showing a significantly lower incidence of CFTR mutations in CBAVD/CUAVD patients (P ≪ 0.0001), compared with that found in the CF patient population. Only three CBAVD patients were found with more than one CFTR mutation (ΔF508/L206W, ΔF508/R74W+D1270N, Rl 17H/712-1G→T), highlighting L206W, R74W/ D1270N, and R117H as benign CF mutations. Sweat electrolyte values were increased in 76.6% of CBAVD patients, but three individuals without CFTR mutations had normal sweat electrolyte levels (10% of the total CBAVD patients), suggesting that factors other than CFTR mutations are involved in CBAVD. The failure to identify a second mutation in exons and their flanking regions of the CFTR gene suggests that these mutations could be located in introns or in the promoter region of CFTR. Such mutations could result in CFTR levels below the minimum 6%–10% necessary for normal protein function.
Similar content being viewed by others
References
Anguiano A, Oates RD, Amos JA, Dean M, Gerrard B, Stewart C, Maher TA, White MB, Milunsky A (1992) Congenital bilateral absence of the vas deferens: a primarily genital form of cystic fibrosis. JAMA 267: 1794–1797
Audrézet MP, Mercier B, Guillermit H, Quéré I, Verlingue C, Rault G, Férec C (1993) Identification of 12 novel mutations in the CFTR gene. Hum Mol Genet 2: 51–54
Boat TF, Welsh MJ, Beaudet AL (1989) Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic basis of inherited disease, 6th edn. McGraw-Hill, New York, pp 2649–2680
Casals T, Nunes V, Palacio A, Giménez J, Gaona A, Ibáñez N, Morral N, Estivill X (1993) Cystic fibrosis in Spain: high frequency of mutation G542X in the Mediterranean coastal area. Hum Genet 91: 66–70
Cheng S, Gregory R, Marshall J, Paul S, Souza D, White G, O'Riordan C, et al (1990) Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63: 827–837
Chillón M, Casals T, Nunes V, Giménez J, Pérez Ruiz E, Estivill X (1993) Identification of a new missense mutation (P205S) in the first transmembrane domain of the CFTR gene associated with a mild cystic fibrosis phenotype. Hum Mol Genet 10: 1741–1742
Chillón M, Casals T, Giménez J, Nunes V, Estivill X (1994a) Analysis of the CFTR gene in the Spanish population: SSCP-screening for 60 known mutations and identification of four new mutations (Q30X, A120T, 1812–1G>A and 3667de14). Hum Mutat 3: 223–230
Chillón M, Casals T, Giménez J, Ramos MD, Palacio A, Morral N, Estivill X, Nunes V (1994b) Analysis of the CFTR gene confirms the high genetic heterogeneity of the Spanish population: 43 mutations account for only 78% of CF chromosomes. Hum Genet 93: 447–451
Chu C-S, Trapnell BC, Curristin SM, Cutting GR, Crystal RG (1992) Extensive post-transcriptional deletion of the coding sequences for part of nucleotide-binding fold 1 in respiratory epithelial mRNA transcripts of the CFTR gene is not associated with the clinical manifestations of cystic fibrosis. J Clin Invest 90: 785–790
Claustres M, Laussel M, Desgeorges M, Giansily M, Culard JF, Razakatsara G, Demaille J (1993) Analysis of the 27 exons and flanking regions of the cystic fibrosis gene: 40 different mutations account for 91. 2% of the mutant alleles in Southern France. Hum Mol Genet 8: 1209–1213
Culard JF, Desgeorges M, Costa P, Laussel M, Razakatzara G, Navratil H, Demaille J, Claustres M (1994) Analysis of the whole CFTR coding regions and splice junctions in azoospermic men with congenital bilateral aplasia of epididymis or vas deferens. Hum Genet 93: 467–470
Dean M, White M, Amos J, Gerrard B, Stewart C, Khaw KT, Leppert M (1990) Multiple mutations in highly conserved residues are found in mildly affected cystic fibrosis patients. Cell 61: 863–870
Dumur V, Gervais R, Rigot JM, Lafitte JJ, Manouvrier S, Biserte J, Mazeman E, Roussel P (1990) Abnormal distribution of CF ΔF508 allele in azoospermic men with congenital aplasia of the epididymis and vas deferens. Lancet 336: 512
Estivill X, Ortigosa L, Pérez-Frias J, Dapena J, Ferrer J, Peña J, Peña L, Llevadot R, Nunes V, Cobos N, Vázquez C, Casals T (1995) Clinical characteristics of 16 cystic fibrosis patients with the missense mutation R334W, a pancreatic insufficiency mutation with variable age of onset and interfamilial clinical differences. Hum Genet (in press)
Fanen P, Ghanem N, Vidaud M, Besmond C, Martin J, Costes B, Plassa F, Goossens M (1992) Molecular characterization of cystic fibrosis: 16 novel mutations identified by analysis of the whole cystic fibrosis transmembrane conductance regulator (CFTR) coding regions and splice site junctions. Genomics 13: 770–776
Gervais R, Dumur V, Rigot JM, Lafitte JJ, Roussel P, Claustres M, Demaille J (1993) High frequency of the R117H cystic fibrosis mutation in patients with congenital absence of the vas deferens. N Engl J Med 328: 446–447
Gibson LE, Cooke RE (1959) A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 23: 545
Heaton ND, Pryor JP (1990) Vasa aplasia and cystic fibrosis. Br J Urol 66: 538–540
Holsclaw DS, Perlmutter AD, Jockin H, Shwachman H (1971) Genital abnormalities in male patients with cystic fibrosis. J Urol 106: 568–574
Jequier AM, Ansell ID, Bullimore NJ (1985) Congenital absence of the vasa deferentia presenting with infertility. J Androl 6: 15–19
Johnson LG, Olsen JC, Sarkadi B, Moore KL, Swanstrom R, Boucher RC (1992) Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nature Genet 2: 21–25
Kaplan E, Shwachman H, Perlmutter AD, Rule A, Khaw K-T, Holsclaw DS (1968) Reproductive failure in males with cystic fibrosis. N Engl J Med 279: 65–69
Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui L-C (1989) Identification of the cystic fibrosis gene: genetic analysis. Science 245: 1073–1080
Morral N, Estivill X (1992) Multiplex PCR amplification of three microsatellites within the CFTR gene. Genomics 13: 1362–1364
Morral N, Nunes V, Casals T, Chillón M, Giménez J, Bertranpetit J, Estivill X (1993) Microsatellite haplotypes for cystic fibrosis: mutation frameworks and evolutionary tracers. Hum Mol Genet 7: 1015–1022
Nunes V, Chillon M, Dörk T, Tümmler B, Casals T, Estivill X (1993) A new missense mutation (E92 K) in the first transmembrane domain of the CFTR gene causes a benign cystic fibrosis phenotype. Hum Mol Genet 1: 79–80
Oates RD, Amos JA (1994) The genetic basis of congenital bilateral absence of the vas deferens and cystic fibrosis. J Androl 15: 1–8
Osborne LR, Lynch M, Middleton PG, Alton EWFW, Geddes DM, Pryor JP, Hodson ME, Santis GK (1993) Nasal epithelial ion transport and genetic analysis of infertile men with congenital bilateral absence of the vas deferens. Hum Mol Genet 2: 1605–1609
Pritchard DJ (1991) Cystic fibrosis allele frequency, sex ratio anomalies and fertility: a new theory for the dissemination of mutant alleles. Hum Genet 87: 671–676
Rigot JM, Lafitte JJ, Dumur V, Gervais R, Manouvrier S, Biserte J, Mazeman E, Roussel P (1991) Cystic fibrosis and congenital absence of the vas deferens. N Engl J Med 325: 64–65
Silber SJ, Patrizio P, Asch RH (1990) Quantitative evaluation of testicular histology in men with congenital absence of the vas deferens undergoing epididymal sperm aspiration. Hum Reprod 5: 89–93
Strong TV, Smit LS, Turpin SV, Cole JL, Tom Hon C, Petty TL, Craig MW, Rosenaw EC, Tsui L-C, Iannuzzi MC, Knowles MR, Collins FS (1991) Cystic fibrosis mutation in two sisters with mild disease and normal sweat electrolyte levels. N Engl J Med 325: 1630–1634
The Cystic Fibrosis Genotype/Phenotype Consortium (1993) Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med 28: 1308–1313
Tizzano EF, Chitayat D, Buchwald M (1993) Cell-specific localization of CFTR mRNA shows developmentally regulated expression in human fetal tissues. Hum Mol Genet 2: 219–224
Trezise AEO, Chambers JA, Wardle CJ, Gould S, Harris A (1993) Expression of the cystic fibrosis gene in human foetal tissues. Hum Mol Genet 2: 213–218
Tsui L-C (1992) Mutations and sequence variations detected in the cystic fibrosis transmembrane conductance regulator (CFTR) gene: a report from the Cystic Fibrosis Genetic Analysis Consortium. Hum Mutat 1: 197–203
Tucker SJ, Tannahill D, Higgins CF (1992) Identification and developmental expression of the Xenopus laevis cystic fibrosis transmembrane conductance regulator gene. Hum Mol Genet 1: 77–82
Zielenski J, Rozmahel R, Bozon D, Kerem B, Grzelczak Z, Riordan J, Rommens JM, Tsui L-C (1991) Genomic DNA sequence of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Genomics 10: 214–228
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Casals, T., Bassas, L., Ruiz-Romero, J. et al. Extensive analysis of 40 infertile patients with congenital absence of the vas deferens: in 50% of cases only one CFTR allele could be detected. Hum Genet 95, 205–211 (1995). https://doi.org/10.1007/BF00209403
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00209403