Abstract
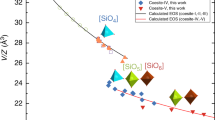
The crystal structure of ScAlO3 has been refined at temperatures up to 1100° C on the basis of x-ray powder diffraction data. The thermal expansion is adequately described by a Grüneisen-Debye model with the elastic Debye temperature and an effective Grüneisen parameter of 1.6. The volumetric thermal expansion of 3.0% between 10 and 1100° C, corresponding to a mean thermal expansion coefficient of 2.7 × 10−5 K−1, is entirely attributable to the expansion of the AlO6 octahedra. The interoctahedral angles, though not fixed by symmetry, do not vary significantly with temperature —indicating that the expansivities of the constituent AlO6 and distorted ScO8 polyhedra are well matched. Similar considerations of polyhedral expansivity suggest thermal expansion coefficients of ∼2 × 10−5K−1 for cubic CaSiO3 perovskite and a value between 2 × 10−5 K−1 and 4 × 10−5 K−1 for MgSiO3 perovskite. The lower value is consistent with the reconnaissance measurements for Mg0.9Fe0.1SiO3 (Knittle et al. 1986) below 350° C, with low-temperature measurements of single-crystal MgSiO3 (Ross and Hazen 1989), and with the results of some recent calculations. The markedly greater expansivity ∼4 × 10−5 K−1 measured at higher temperatures (350–570° C) by Knittle et al. is inconsistent with the simple Grüneisen-Debye quasiharmonic model and may reflect the marginal metastability of the orthorhombic perovskite phase. Under these circumstances, extrapolation of the measured expansivity is hazardous and may result in the under-estimation of lower mantle densities and the drawing of inappropriate inferences concerning the need for chemical stratification of the Earth's mantle.
Similar content being viewed by others
References
Anderson OL (1988) A ferroelectric transition in the lower mantle? EOS (Trans Am Geophys Union) 69:1451
Anderson OL, Schreiber E, Liebermann RC, Soga N (1968) Some elastic constant data on minerals relevant to geophysics. Rev Geophys 6:491–524
Bass JD (1984) Elasticity of single-crystal SmAlO3, GdAlO3 and ScAlO3 perovskites. Phys Earth Planet Inter 36:145–156
Brown ID, Altermatt D (1985) Bond valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Crystallogr B41:244–247
Cohen RE (1987) Elasticity and equation of state of MgSiO3 perovskite. Geophys Res Lett 14:1053–1056
Hazen RM, Finger LW (1982) Comparative crystal chemistry: temperature, pressure, composition and the variation of crystal structure. Wiley, New York, pp 115–146
Hazen RM, Prewitt CT (1977) Effects of temperature and pressure on interatomic distances in oxygen-based minerals. Am Mineral 62:309–315
Hemley RJ, Jackson MD, Gordon RG (1987) Theoretical study of the structure, lattice dynamics and equations of state of perovskite-type MgSiO3 and CaSiO3. Phys Chem Minerals 14:2–12
Hemley RJ, Cohen RE, Yeganeh-haeri A, Mao HK, Weidner DJ (1989) Raman spectroscopy and lattice dynamics of MgSiO3-perovskite at high pressure. In: Navrotsky A, Weidner DJ (eds) Perovskite: a structure of great interest of geophysics and materials science. AGU (Washington), pp 35–44
Hill RJ (1984) X-ray powder diffraction profile refinement of synthetic hercynite. Am Mineral 69:937–942
Hill RJ, Howard CJ (1986) A computer program for Rietveld analysis of fixed wavelength x-ray and neutron powder diffraction patterns. Aust. Atomic Energy Comm. (now ANSTO) Report No. M112, 14pp, Lucas Heights Res. Lab., Menai, New South Wales, Australia
Horiuchi H, Ito E, Weidner DJ (1987) Perovskite-type MgSiO3: single-crystal x-ray diffraction study. Am Mineral 72:357–360
International Tables for X-ray Crystallography (1974) Vol. IV. Kynoch Press, Birmingham (Present distributor D. Reidel, Dordrecht)
Ito H, Kawada K, Akimoto S (1974) Thermal expansion of stishovite. Phys Earth Planet Inter 8:277–281
Jackson I (1983) Some geophysical constraints on the chemical composition of the Earth's lower mantle. Earth Planet Sci Lett 62:91–103
Jeanloz R, Knittle E (1989) Density and composition of the lower mantle. Philos Trans R Soc London A328: 377–389
Jeanloz R, Thompson AB (1983) Phase transitions and mantle discontinuities. Rev Geophys Space Phys 21:51–74
Jones LEA (1979) Pressure and temperature dependence of the single crystal elastic moduli of the cubic perovskite KMgF3. Phys Chem Minerals 4:23–42
Knittle E, Jeanloz R, Smith GL (1986) Thermal expansion of silicate perovskite and stratification of the Earth's mantle. Nature 319:214–216
Liebermann RC, Jones LEA, Ringwood AE (1977) Elasticity of aluminate, titanate, stannate and germanate compounds with the perovskite structure. Phys Earth Planet Inter 14:165–178
Liu X, Liebermann RC (1988) A2+B4+O3 perovskites at high temperature. EOS (Trans Am Geophys Union) 69:1451
Liu L, Ringwood AE (1975) Synthesis of a perovskite-type polymorph of CaSiO3. Earth Planet Sci Lett 28:209–211
Liu X, Wang Y, Liebermann RC (1988) Orthorhombic-tetragonal phase transition in CaGeO3 perovskite at high temperature. Geophys Res Lett 15:1231–1234
Meagher EP (1975) The crystal structures of pyrope and grossular at elevated temperatures. Am Mineral 60:218–228
Megaw HD (1971) Crystal structures and thermal expansion. Mater Res Bull 6:1007–1018
Navrotsky A (1989) Thermochemistry of perovskites. In: Navrotsky A, Weidner DJ (eds) Perovskite: a structure of great interest to geophysics and materials science. AGU (Washington) pp 67–80
Norrestam P (1969) Refinement of the crystal structure of scandium oxide from single-crystal diffractometer data. Ark Kemi 29:343–349
Reid AF, Ringwood AE (1975) High-pressure modification of ScAlO3 and some geophysical implications. J Geophys Res 80:3363–3370
Rietveld, H.M. (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr 2:65–71
Ross NL, Hazen RM (1989) Single-crystal x-ray diffraction study of MgSiO3 perovskite from 77 to 400 K. Phys Chem Minerals 16:415–420
Sasaki S, Prewitt CT, Liebermann RC (1983) The crystal structure of CaGeO3 perovskite and the crystal chemistry of GdFeO3-type perovskites. Am Mineral 68:1189–1198
Sinclair W, Eggleton RA, Ringwood AE (1979) Crystal synthesis and structure refinement of high-pressure ScAlO3 perovskite. Z Krist 149:307–314
Skinner BJ (1966) Thermal expansion. In: Clark SP Jr (ed) Handbook of Physical Constants. Geological Society of America Memoir, 97. Geol. Soc. Am., New York, pp 75–96
Suzuki I, Ohtani E, Kumazawa M (1979) Thermal expansion of γ-Mg2SiO4. J Phys Earth 27:53–61
Wolf GH, Bukowinski MST (1987) Theoretical study of the structural properties and equations of state of MgSiO3 and CaSiO3 perovskites: implications for lower mantle composition. In: Manghnani MH, Syono Y (eds) High pressure research in mineral physics. Terra (Tokyo)/Amer. Geophys. (Washington), pp 313–331
Wyckoff RWG (1963) Crystal Structures, vol 2. Wiley-Interscience, New York, 588 pp
Yamanaka T, Prewitt CT, Liebermann RC (1987) High temperature study of ScAlO3 orthorhombic perovskite. Mineral Soc Jpn (abstract)
Yeganeh-haeri A, Weidner DJ, Ito E (1989) Elasticity of MgSiO3 in the perovskite structure. Science 243:787–789
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hill, R.J., Jackson, I. The thermal expansion of ScAlO3 — A silicate perovskite analogue. Phys Chem Minerals 17, 89–96 (1990). https://doi.org/10.1007/BF00209229
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00209229