Abstract
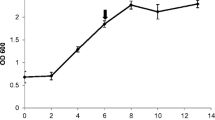
Wounding of plants by insects is often mimicked in the laboratory by mechanical means such as cutting or crushing, and has not been compared directly with other forms of biotic stress such as virus infection. To compare the response of plants to these types of biotic and abiotic stress, trypsin inhibitor (TI) activity induced locally and systemically in mature tobacco (Nicotiana tabacum L.) and tomato (Lycopersicon esculentum L.) plants was followed for 12 days. In tobacco, cutting, crushing and insect feeding all induced comparable levels of TI activity of approx. 5 nmol·(mg leaf protein)−1 in wounded leaves, while tobacco mosaic virus (TMV) infection of tobacco induced 10-fold lower amounts in the infected leaves. In tomato, feeding by insects also led to the induction of a level of TI activity of 5 nmol·(mg leaf protein)−1. In contrast, both cutting and crushing of tomato leaves induced 10-fold higher amounts. These data show that biotic stress, in the form of insect feeding and TMV infection, and abiotic stress, in the form of wounding, have different effects on local levels of induced TI activity in mature tobacco and tomato plants. Irrespective of the type of wounding, in neither tobacco nor tomato could systemic induction of TI activity be observed in nearby unwounded leaves, which suggests that systemic induction of TI activity in mature tobacco and tomato plants is different from systemic TI induction in seedlings. Wounding of tobacco leaves, however, did increase the responsiveness to wounding elsewhere in the plant, as measured by an increased induction of TI activity.
Similar content being viewed by others
Abbreviations
- PI(-1, -2):
-
proteinase inhibitor (I, II)
- SI:
-
subtilisin inhibitor
- TI:
-
trypsin inhibitor
- TMV:
-
tobacco mosaic virus
References
Atkinson, A.H., Heath, R.L., Simpson, R.J., Clarke, A.E., Anderson, M.A. (1993) Proteinase inhibitors in Nicotiana alata stigmas are derived from a precursor protein which is processed into five homologous inhibitors. Plant Cell 5, 203–213
Bender, M.L., Begue-Canton, M.L., Blakeley, R.L., Brubacher, L.J., Feder, J., Gunter, C.R., Kezdy, F.J., Killheffer, J.V, Marshall, T.H., Miller, C.G., Roeske, R.G., Stoops, J.K. (1966) The determination of the concentration of hydrolytic enzyme solutions: α-chymotrypsin, trypsin, papain, elastase, subtilisin, and acetylcholinesterase. J. Am. Chem. Soc. 88, 5890–5913
Bradford, M.N. (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of dye binding. Anal. Biochem. 72, 248–254
Broadway, R.M., Duffey, S.S., Pearce, G., Ryan, C.A. (1986) Plant proteinase inhibitors: A defense against herbivorous insects? Entomol. Exp. Appl. 41, 33–38
Chase, T., Shaw, E. (1967) p-Nitrophenyl-p′-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem. Biophys. Res. Commun. 29, 508–514
Duffey, S.S., Felton, G.W. (1989) Plant enzymes in resistance to insects. In: Biocatalysis in agricultural biotechnology, Symposium Series No. 389, pp. 289–313, Whitaker, J.R., Sonnet, P.E., eds. American Chemical Society, Washington
Farmer, E.E., Ryan, C.A. (1990) Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. USA 87, 7713–7716
Geoffroy, P., Legrand, M., Fritig, B. (1990) Isolation and characterization of a proteinaceous inhibitor of microbial proteinases induced during the hypersensitive reaction of tobacco to Tobacco Mosaic Virus. Mol. Plant-Microbe Interact 3, 327–333
Green, T.R., Ryan, C.A. (1972) Wound-induced proteinase inhibitors in plant leaves: a possible defense mechanism against insects. Science 175, 776–777
Heitz, T., Geoffroy, B., Fritig, B., Legrand, M. (1993) Molecular cloning and expression of an inhibitor of microbial proteinases induced during the hypersensitive reaction of tobacco to TMV. In: Developments of plant physiology, vol. 2, Mechanisms of plant defense responses, pp. 291–294, Fritig, B., Legrand, M., eds. Kluwer Acad. Publ., Dordrecht
Hilder, V.A., Gatehouse, A.M.R., Sheerman, S.E., Barker, R.F., Boulter, D. (1987) A novel mechanism of insect resistance engineered into tobacco. Nature 330, 160–163
Hoffmann, M.P., Zalom, F.G., Wilson, L.T., Smilanick, J.M., Malyj, L.D., Kiser, J., Hilder, V.A., Barnes, W.M. (1992) Field evaluation of transgenic tobacco containing genes encoding Bacillus thuringiensis δ-endotoxin or cowpea trypsin inhibitor: Efficacy against Helicoverpa zea (Lepidoptera: Noctuidae). J. Econ. Entomol. 85, 2516–2522
Johnson, R., Narvaez, J., An, G., Ryan, C.A. (1989) Expression of proteinase inhibitors I and II in transgenic plants: Effects on natural defense against Manduca sexta larvae. Proc. Natl. Acad. Sci. USA 86, 9871–9875
Jongsma, M.A., Bakker, P.L., Stiekema, W.J. (1993) Quantitative determination of serine proteinase inhibitor activity using a radial diffusion assay. Anal. Biochem. 212, 79–84
Kernan, A., Thornburg, R.W. (1989) Auxin levels regulate the expression of a wound-inducible proteinase inhibitor II-chloramphenicol acetyl transferase gene fusion in vitro and in vivo. Plant Physiol. 91, 73–78
Linthorst, H.J.M., Brederode, F.Th., van derDoes, C., Bol, J.P. (1993) Tobacco proteinase inhibitor I genes are locally, but not systemically induced by stress. Plant Mol. Biol. 21, 985–992
Nelson, C.E., Walker-Simmons, M., Makus, D., Zuroske, G., Graham, J., Ryan, C.A. (1983) Regulation of synthesis and accumulation of inhibitors in plants. In: Plant Resistance to insects, pp. 103–122, Hedin, P.A., ed. American Chemical Society, Washington
Pearce, G., Strydom, D., Johnson, S., Ryan, C.A. (1991) A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253, 895–897
Pearce, G., Johnson, S., Ryan, C.A. (1993) Purification and characterization from tobacco (Nicotiana tabacum) leaves of six small, wound-inducible, proteinase isoinhibitors of the potato inhibitor II family. Plant Physiol. 102, 639–644
Ryan, C.A. (1978) Proteinase inhibitors in plant leaves: A biochemical model for pest-induced natural plant protection. Trends Biochem. Sci. 3, 148–150
Ryan, C.A. (1992) The search for the proteinase inhibitor-inducing factor, PIIF. Plant Mol. Biol. 19, 123–133
Thornburg, R., Li, X. (1991) Wounding Nicotiana tabacum leaves causes a decline in endogenous indole-3-acetic acid. Plant Physiol. 96, 802–805
Wildon, D.C., Thain, J.F., Minchin, P.E.H., Gubb, I.R., Reilly, A.J., Skipper, Y.D., Doherty, H.M., O'Donnell, P.J., Bewles, D.J. (1992) Electrical signalling and systemic proteinase inhibitor induction in the wounded plant. Nature 360, 62–65
Wolfson, J.L., Murdock, L.L. (1990) Growth of Manduca sexta on wounded tomato plants: role of induced proteinase inhibitors. Entomol. Exp. Appl. 54, 257–264
Author information
Authors and Affiliations
Additional information
We wish to thank Johan Hulsman and Hans Janssen for taking excellent care of the plants in the greenhouse and Jan Peter Nap for assisting the computer analysis of the data. Ruud de Maagd (CPRO-DLO) and Ab van Kammen (Molecular Biology Dept., Agricultural Univ. Wageningen, Wageningen, The Netherlands) are thanked for their critical comments on the manuscript.
Rights and permissions
About this article
Cite this article
Jongsma, M.A., Bakker, P.L., Visser, B. et al. Trypsin inhibitor activity in mature tobacco and tomato plants is mainly induced locally in response to insect attack, wounding and virus infection. Planta 195, 29–35 (1994). https://doi.org/10.1007/BF00206288
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00206288