Abstract
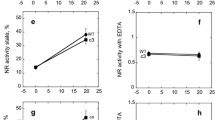
Nitrate reductase in spinach (Spinacia oleracea L.) leaves was rapidly inactivated in the dark and reactivated by light, whereas in pea (Pisum sativum L.), roots, hyperoxic conditions caused inactivation, and anoxia caused reactivation. Reactivation in vivo, both in leaves and roots, was prohibited by high concentrations (10–30 μM) of the serine/threonine-protein phosphatase inhibitors okadaic acid or calyculin, consistent with the notion that protein dephosphorylation catalyzed by type-1 or type-2A phosphatases was the mechanism for the reactivation of NADH-nitrate reductase (NR). Following inactivation of leaf NR in vivo, spontaneous reactivation in vitro (in desalted extracts) was slow, but was drastically accelerated by removal of Mg2+ with excess ethylenediaminetetraacetic acid (EDTA), or by desalting in a buffer devoid of Mg2+. Subsequent addition of either Mg2+, Mn2+ or Ca2+ inhibited the activation of NR in vitro. Reactivation of NR (at pH 7.5) in vitro in the presence of Mg2+ was also accelerated by millimolar concentrations of AMP or other nucleoside monophosphates. The EDTA-mediated reactivation in desalted crude extracts was completely prevented by protein-phosphatase inhibitors whereas the AMP-mediated reaction was largely unaffected by these toxins. The Mg2+-response profile of the AMP-accelerated reactivation suggested that okadaic acid, calyculin and microcystin-LR were rather ineffective inhibitors in the presence of divalent cations. However, with partially purified enzyme preparations (5–15% polyethyleneglycol fraction) the AMPmediated reactivation was also inhibited (65–80%) by microcystin-LR. Thus, the dephosphorylation (activation) of NR in vitro is inhibited by divalent cations, and protein phosphatases of the PP1 or PP2A type are involved in both the EDTA and AMP-stimulated reactions. Evidence was also obtained that divalent cations may regulate NR-protein phosphatase activity in vivo. When spinach leaf slices were incubated in Mg2+ -and Ca2+-free buffer solutions in the dark, extracted NR was inactive. After addition of the Ca2+ /Mg2+-ionophore A 23187 plus EDTA to the leaf slices, NR was activated in the dark. It was again inactivated upon addition of divalent cations (Mg2+ or Ca2+). It is tentatively suggested that Mg2+ fulfills several roles in the regulatory system of NR: it is required for active NR-protein kinase, it inactivates the protein phosphatase and is, at the same time, necessary to keep phospho-NR in the inactive state. The EDTA- and AMP-mediated reactivation of NR in vitro had different pH optima, suggesting that two different protein phosphatases may be involved. At pH 6.5, the activation of NR was relatively slow and the addition or removal of Mg2+ had no effect. However, 5′-AMP was a potent activator of the reaction with an apparent K m of 0.5 mM. There was also considerable specificity for 5′AMP relative to 3′- or 2′-AMP or other nucleoside monophoposphates. We conclude that, depending upon conditions, the signals triggering NR modulation in vivo could be either metabolic (e.g. 5′-AMP) or physical (e.g. cytosolic [Mg2+]) in nature.
Similar content being viewed by others
Abbreviations
- DTT:
-
dithiothreitol
- Mops:
-
3-(N-morpholino)propanesulfonic acid
- NR:
-
NADH-nitrate reductase
- NRA:
-
nitrate-reductase activity
- PP:
-
protein phosphatase
References
Biojolan, C., Takai, A. (1988) Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Biochem. J. 256, 283–290
Glaab, J., Kaiser, W.M. (1993) Rapid modulation of nitrate reductase in pea roots. Planta 191, 173–179
Hagemann, R.H., Reed, A.J. (1980) Nitrate reductase from higher plants. Methods Enzymol. 69, 270–280
Hardie, D.G. (1993) Use of protein phosphatase inhibitors in intact cells, in: Protein phosphorylation. A practical approach, pp. 109–120, D.G. Hardie, ed. Oxford University Press, New York
Huber, J.L., Huber, S.C., Redinbaugh, M.G., Campbell, W.H. (1992) Reversible light/dark modulation of spinach leaf nitrate reductase activity involves protein phosphorylation. Arch. Biochem. Biophys. 296, 58–65
Huber, J.L., Huber, S.C., McMichael, R.W. Jr., Redinbaugh, M.G., Campbell, W.H. (1993) Modulation of nitrate reductase by protein phosphorylation. Curr. Top. Plant Biochem. Physiol. 12, 7–8
Huber, S.C., Huber, J.L., McMichael, R.W, Campbell, W.H., Redinbaugh, M.G. (1992) Regulation of cytoplasmatic C- and N-metabolism by protein phosphorylation. In: Research in photosynthesis, vol. III, pp. 675–682, Murata, N., ed. Kluwer Academic Publishers, The Netherlands
Kaiser, W.M., Brendle-Behnisch, E. (1991) Rapid modulation of spinach leaf nitrate reductase activity by photosynthesis. I. Modulation in vivo by CO2-availability. Plant Physiol. 96, 363–367
Kaiser, W.M., Spill, D. (1991) Rapid modulation of spinach leaf nitrate reductase activity by photosynthesis II. In vitro-modulation by ATP and AMP. Plant Physiol. 96, 368–375
Kaiser, W.M., Spill, D., Brendle-Behnisch, E. (1992) Adenine nucleotides are apparently involved in the light-dark modulation of spinach leaf nitrate reductase. Planta 186, 236–240
MacKintosh, C. (1992) Regulation of spinach leaf nitrate reductase. Biochim. Biophys. Acta 1137, 121–126
MacKintosh, R.W, MacKintosh, C. (1993) Regulation of plant metabolism by reversible protein (serine/threonine) phosphorylation. In: Posttranslational modifications in plants, pp. 197–212, (Society for experimental biology. Seminar series 53) Battey, N.H., Dickinson, H.G., Hetherington, A.M., eds. Cambridge University Press
Reggiani, R., Mattana, M., Aurisano, N., Bertani, A. (1993) Utilization of stored nitrate during the anaerobic germination of rice seeds. Plant Cell Physiol. 34, 379–383
Riens, B., Heldt, H.W. (1992) Decrease of nitrate reductase activity in spinach leaves during a light-dark transition. Plant Physiol. 98, 573–577
Solomonson, L.P., Barber, M.J. (1990) Assimilatory nitrate reductase: functional properties and regulation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41, 225–253
Spill, D., Kaiser, W.M. (1994) Partial purification of two proteins (100 kDa and 67 kDa), cooperating in the ATP-dependent inactivation of spinach leaf nitrate reductase. Planta 192, 183–188
Weiner, H., MacMichael, R.W, Huber, S.C. (1992) Identification of factors regulating the phosphorylation status of sucrose-phosphate synthase in vivo. Plant Physiol. 99, 1435–1442
Author information
Authors and Affiliations
Additional information
This paper is dedicated to Prof. O.K. Volk on the occasion of his 90th birthday
The skilled technical assistance of Elke Brendle-Behnisch is gratefully acknowledged. The investigations were cooperatively supported by the Deutsche Forschungsgemeinschaft (SFB 251), the U.S. Department of Agriculture, Agricultural Research Services, Raleigh, NC. This work was also supported in part by a grant from the U.S. Department of Energy (Grant DE-A I05-91 ER 20031 to S.C.H.).
Rights and permissions
About this article
Cite this article
Kaiser, W.M., Huber, S. Modulation of nitrate reductase in vivo and in vitro: Effects of phosphoprotein phosphatase inhibitors, free Mg2+ and 5′-AMP. Planta 193, 358–364 (1994). https://doi.org/10.1007/BF00201813
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00201813