Abstract
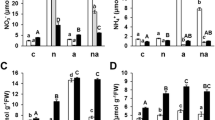
One cytosolic glutamine synthetase (GS, EC 6.3.1.2) isoform (GS 1a) was active in the germinating seeds of barley (Hordeum vulgare L.). A second cytosolic GS isoform (GS 1b) was separated from the leaves as well as the roots of 10-d-old seedlings. The chloroplastic isoform (GS 2) was present and active only in the leaves. The three GS isoforms were active in N-supplied (NH+ 4 or NO −3 ) as well as in N-free-grown seedlings. This indicates (i) that a supply of nitrogen to the germinating seeds was not necessary for the induction of the GS isoforms and (ii) that no nitrogen-specific isoforms appeared during growth of seedlings with different nitrogen sources. The activity of GS, however, depended on the seedlings' nitrogen source: the specific activity was much higher in the leaves and much lower in the roots of NH+ 4-grown barley than in the respective organs of NO −3 -fed or N free-grown plants. With increasing concentrations of NH+ 4 (supplied hydroponically during growth), the specific activity of GS 1b increased in the leaves, but decreased in the roots. The activity of GS 2 (leaf) also increased with increasing NH+ 4 supply, whereas GS 1a activity (leaf and root) was not affected. The changes in the activities of GS 1b and GS 2 were correlated with changes in the subunit compositions of the active holoenzymes: growth at increased levels of external NH+ 4 resulted in an increased abundance of one of the four GS subunits, and of two of the five GS 1b subunits in the leaves. In the roots, however, the abundance of these two GS 1b subunits was decreased under the same growth conditions, indicating an organ-specific difference either in the expression of the genes coding for the respective GS 1b subunits or in the assembly of the GS 1b holoenzymes. Furthermore, growth at different levels of NH+ 4 resulted in changes in the substrate affinities of the isoforms GS 1b (root and leaf) and GS 2 (leaf), presumably due to the changes in the subunit compositions of the active holoenzymes.
Similar content being viewed by others
Abbreviations
- FPLC:
-
fast protein liquid chromatography
- GHA:
-
γ-glutamyl hydroxamate
- GS:
-
glutamine synthetase
References
Avni, A., Edelman, M., Rachailovich, I., Aviv, D., Fluhr, R. (1989) A point mutation in the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase affects holoenzyme assembly in Nicotiana tabacum. EMBO J. 8, 1915–1918
Bennet, M.J., Cullimore, J.V. (1989) Glutamine synthetase isoenzymes of Phaseolus vulgaris L.: subunit composition in developing root nodules and plumules. Planta 179, 433–440
Blackwell, R.D., Murray, A.J.S., Lea, P.J. (1987) Inhibition of photosynthesis in barley with decreased levels of chloroplastic glutamine synthetase activity. J. Exp. Bot. 38, 1799–1809
Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254
Brangeon, J., Nato, A., Forchioni, A. (1989) Ultrastructural detection of ribulose-1,5-bisphosphate carboxylase protein and its subunit mRNAs in wild-type and holoenzyme-deficient Nicotiana using immuno-gold and in situ-hybridization techniques. Planta 177, 151–159
Chourey, P.S., Latham, M.D., Still, P.E. (1986) Expression of two sucrose synthase genes in endosperm and seedlings of maize: evidence of tissue specific polymerisation of protomers. Mol. Gen. Genet. 203, 251–255
Cock, J.M., Mould, R.M., Bennet, M.J., Cullimore, J.V. (1990) Expression of glutamine synthetase in roots and nodules of Phaseolus vulgaris following changes in the ammonium supply and infection with various Rhizobium mutants. Plant Mol. Biol. 14, 549–560
Cock, J.M., Brock, I.W., Watson, A.T., Swarup, R., Morby, A.P., Cullimore, J.V. (1991) Regulation of glutamine synthetase genes in leaves of Phaseolus vulgaris. Plant Mol. Biol. 17, 716–771
Cullimore, J.V., Miflin, B.J. (1984) Immunological studies on glutamine synthetase using antisera to the two plant forms of the enzyme from Phaseolus vulgaris root nodules. J. Exp. Bot. 35, 581–587
Dávila, G., Lara, M., Guzmán, J., Mora, J. (1980) Relation between structure and function of Neurospora crassa glutamine synthetase. Biochem. Biophys. Res. Commun. 92, 134–140
De la Haba, P., Cabello, P., Maldonado, J.M. (1992) Glutamine-synthetase isoforms appearing in sunflower cotyledons during germination. Planta 186, 577–581
Dunbar, B.S., Kimura, H., Timmons, T.M. (1990) Protein analysis using high-resolution two-dimensional polyacrylamide gel electrophoresis. Methods Enzymol. 182, 441–459
Edwards, J.W., Walker, E.L., Coruzzi, G.M. (1990) Cell-specific expression in transgenic plants reveals nonoverlapping roles for chloroplast and cytosolic glutamine synthetase. Proc. Natl. Acad. Sci. USA 87, 3459–3463
Fentem, P.A., Lea, P.J., Stewart, G.R. (1983) Ammonium assimilation in the roots of nitrate and ammonia grown Hordeum vulgare (cv. Golden promise). Plant Physiol. 71, 496–501
Forde, B.G., Day, H.M., Turton, J.F., Wen-jun, S., Cullimore, J.V., Oliver, J.E. (1989) Two glutamine synthetase genes from Phaseolus vulgaris L. display contrasting developmental and spatial patterns of expression in transgenic Lotus corniculatus plants. Plant Cell 1, 391–401
Freeman, J., Marquez, A.J., Wallsgrove, R.M., Saarelainen, R., Forde, B.G. (1990) Molecular analysis of barley mutants deficient in chloroplast glutamine synthetase. Plant Mol. Biol. 14, 297–311
Hayakawa, T., Kamachi, K., Oikawa, M., Ojima, K., Yamaya, T. (1990) Response of glutamine synthetase isoforms to nitrogen sources in rice cell cultures. Plant Cell Physiol. 31, 1071–1077
Hirel, B., Weatherley, C., Cretin, C., Bergounioux, C., Gadal, P. (1984) Multiple subunit composition of chloroplastic glutamine synthetase of Nicotiana tabacum L. Plant Physiol. 74, 448–450
Hirel, B., Bouet, C., King, B., Layzell, D., Jacobs, F., Verma, D.P.S. (1987) Glutamine synthetase genes are regulated by ammonia provided externally or by symbiotic nitrogen fixation. EMBO J. 5, 1167–1171
Hoelzle, I., Finer, J.J., McMullen, M.D., Streeter, J.G. (1992) Induction of glutamine synthetase activity in nonnodulated roots of Glycine max, Phaseolus vulgaris, and Pisum sativum. Plant Physiol. 100, 525–528
Kozaki, A., Sakamoto, A., Tanaka, K., Takeba, G. (1991) Promotor of the gene for glutamine synthetase from rice shows organspecific and substrate-induced expression in transgenic tobacco plants. Plant Cell Physiol. 32, 353–358
Kozaki, A., Sakamoto, A., Takeba, G. (1992) The promotor of the gene for plastidic glutamine synthetase (GS 2) from rice is developmentally regulated and exhibits substrate-induced expression in transgenic tobacco plants. Plant Cell Physiol. 33, 233–238
Kung, S.D., Sakano, K., Wildman, S.G. (1974) Multiple peptide composition of the large and small subunits of Nicotiana tabacum fraction I protein ascertained by fingerprinting and electrofocusing. Biochim. Biophys. Acta 365, 138–147
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685
Lewis, O.A.M., James, D.M., Hewitt, E.J. (1982) Nitrogen assimilation in barley (Hordeum vulgare L. cv. Mazurka) in response to nitrate and ammonium nutrition. Ann. Bot. 49, 39–49
Lewis, O.A.M., Chadwick, S., Withers, J. (1983) The assimilation of ammonium by barley roots. Planta 159, 483–486
Lightfoot, A., Green, N.K., Cullimore, J.V. (1988) The chloroplastlocated glutamine synthetase of Phaseolus vulgaris L.: nucleotide sequence, expression in different organs and uptake into isolated chloroplasts. Plant Mol. Biol. 11, 191–202
Mäck, G. (1988) Untersuchungen zum Stickswtoffmetabolismus in Zuckerrübenpflanzen unterschiedlicher Entwicklungsstadien. Dissertation, University of Göttingen, Germany
Mäck, G., Tischner, R. (1990a) Glutamine synthetase oligomers and isoforms in sugarbeet (Beta vulgaris L.). Planta 181, 10–17
Mäck, G., Tischner, R. (1990b) The effect of endogenous and externally supplied nitrate on nitrate uptake and reduction in sugarbeet seedlings. Planta 182, 169–173
Mann, A.F., Fentem, P.A., Stewart, G.R. (1979) Identification of two forms of glutamine synthetase in barley (Hordeum vulgare.) Biochem. Biophys. Res. Commun. 88, 515–521
Marsolier, M.C. (1992) Etude de l'expression des gènes codant pour la glutamine synthetase cytosolic chez le soja (Glycine max L.). Doctoral thesis. University of Paris-Sud, Centre D'Orsay, France
Marttila, S., Saarelainen, R., Porali, I., Mikkonen, A. (1993) Glutamine synthetase isoenzymes in germinating barley seeds. Physiol. Plant. 88, 612–618
Miao, G.H., Hirel, B., Marsolier, M.C., Ridge, R.W., Verma, D.P. (1991) Ammonia-regulated expression of a soybean gene encoding cytosolic glutamine synthetase in transgenic Lotus corniculatus. Plant Cell 3, 11–22
Miflin, B.J., Lea, P.J. (1980) Ammonia assimilation. In: The biochemistry of plants, vol. 5, pp. 169–202, Miflin, B.J., ed. Acad. Press, New York
O'Neal, D., Joy, K.W. (1973) Glutamine synthetase of pea leaves. Purification, stabilization, and pH optima. Arch. Biochem. Biophys. 159, 113–122
Ratajczak, L., Ratajczak, W., Mazurowa, H. (1981) The effect of different carbon and nitrogen sources on the activity of glutamine synthetase and glutamate dehydrogenase in lupin embryonic axes. Physiol. Plant. 51, 277–280
Redinbaugh, M.G., Campbell, W.C. (1993) Glutamine synthetase and ferredoxin-dependent glutamate synthase expression in the maize (Zea mays) root primary response to nitrate. Plant Physiol. 101, 1249–1255
Sato, N. (1989) Nucleotide sequence and expression of the phytochrome gene in Pisum sativum: differential regulation by light of multiple transcripts. Plant Mol. Biol. 11, 697–710
Schmidt, G.W., Mishkind, M.L. (1983) Rapid degradation of unassembled ribulose-1,5-bisphosphate carboxilase small subunits in chloroplasts. Proc. Natl. Acad. Sci. USA 86, 886–890
Spreitzer, R.J., Goldschmidt-Clermont, M., Rahire, M., Rochaix, J.-D., Ogren, W.L. (1985) Nonsense mutation in the Chlamydomonas chloroplast gene that codes for the large subunit of ribulose bisphosphate carboxilase/oxygenase in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 82, 5460–5464
Tomizawa, K.-I., Sato, N., Furuya, M. (1989) Phytochrome control of multiple transcripts of the phytochrome gene in Pisum sativum. Plant Mol. Biol. 12, 295–299
van Tunen, A.J., Hartman, S.A., Mur, L.A., Mol, J.N.M. (1989) Regulation of chalcone flavonone isomerase (CHI) gene expression in Petunia hybrida: the use of alternative promotors in corolla, anthers and pollen. Plant Mol. Biol. 12, 539–551
Vézina, L.P., Langlois, J.R. (1989) Tissue and cellular distribution of glutamine synthetase in roots of pea (Pisum sativum) seedlings. Plant Physiol. 90, 1129–1133
Walker, E.L., Coruzzi, G.M. (1989) Developmentally regulated expression of the gene family for cytosolic glutamine synthetase in Pisum sativum. Plant Physiol. 91, 702–708
Wallsgrove, R.M., Turner, J.C., Hall, N.P., Kendall, A.C., Bright, S.W.J. (1987) Barley mutants lacking chloroplast glutamine synthetase — biochemical and genetic analysis. Plant Physiol. 83, 155–158
Author information
Authors and Affiliations
Additional information
Dr. Roger Wallsgrove's (Rothamsted Experimental Station, Harpenden, UK) generous gift of GS antiserum is greatly appreciated.
Rights and permissions
About this article
Cite this article
Mäck, G. Organ-specific changes in the activity and subunit composition of glutamine-synthetase isoforms of barley (Hordeum vulgare L.) after growth on different levels of NH+ 4 . Planta 196, 231–238 (1995). https://doi.org/10.1007/BF00201379
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00201379