Abstract
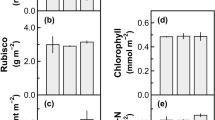
J.M. Keller et al. (1989, EMBO J. 8, 1005–1012) introduced a phytochrome gene controlled by a cauliflower mosaic virus 35S promoter into tobacco (Nicotiana tabacum L.) providing material to test whether several photosynthesis enzymes can be increased by one modification to the plant. We report here that this transgenic tobacco had greater amounts of all enzymes examined as well as greater amounts of total protein and chlorophyll per unit leaf area. Fructose bisphosphatase (E.C. 3.1.3.11), glyceraldehyde 3-phosphate dehydrogenase (E.C. 1.2.1.12), and sucrose-phosphate synthase (E.C. 2.4.1.14) were also higher when expressed per unit protein. However, ribulose-1,5-bisphosphate carboxylase (E.C. 4.1.1.39) amount per unit leaf protein was the same in transgenic and wild-type (WT) plants. Photosynthesis in the transgenic plants was lower than in WT at air levels of CO2, but higher than in WT above 1000 μbar CO2. The photosynthesis results indicated a high resistance to CO2 diffusion in the mesophyll of the transgenic plants. Examination of electron micrographs showed that chloroplasts in the transgenic plants were often cup-shaped, preventing close association between chloroplast and cell surface. Chloroplast cupping may have caused the increase in the mesophyll resistance to CO2 diffusion. We conclude that it is possible to affect more than one enzyme with a single modification, but unexpected physical modifications worsened the photosynthetic performance of this plant.
Similar content being viewed by others
Abbreviations
- CABP:
-
2-carboxyarabitinol 1,5-bisphosphate
- FBP:
-
fructose-1,6-bisphosphate
- FBPase:
-
fructose-1,6-bisphosphatase
- GAP:
-
glyceraldehyde 3-phosphate
- Rubisco:
-
ribulose-1,5-bisphosphate carboxylase
- SPS:
-
sucrose-phosphate synthase
- WT:
-
wild type
References
Barnett, L.K., Clugston, C.K., Jenkins, G.I. (1987) Two phytochrome-mediated effects of light on transcription of genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase-oxygenase in dark-grown pea (Pisum sativum) plants. FEBS Lett. 2, 287–290
Björkman, O. (1981) Responses to different quantum flux densities. In: Encyclopedia of plant physiology, N.S. vol. 12A: Physiological plant ecology I-Responses to the physical environment, pp.57–107, Lange, O.L., Nobel, P.S., Osmond, C.B., Zeigler, H., eds. Springer, Berlin Heidelberg
Bongi, G., Loreto, F. (1989) Gas-exchange properties of saltstressed olive (Oka europa L.) leaves. Plant Physiol. 90, 1408–1416
Cardon, Z.G., Mott, K.A. (1989) Evidence that ribulose 1,5-bis-phosphate (RuBP) binds to inactive sites of RuBP carboxylase in vivo and an estimate of the rate constant for dissociation. Plant Physiol. 89, 1253–1257
Chao, S., Raines, C.A., Longstaff, M., Sharp, P.J., Gale, M.D., Dyer, T.A. (1989) Chromosomal location and copy number in wheat and some of its close relatives of genes for enzymes involved in photosynthesis. Mol. Gen. Gen. 218, 423–430
Cherry, J., Hershey, H.P., Vierstra, R.D. (1991) Characterization of tobacco expressing functional oat phytochrome: Domains responsible for rapid degradation of PFR are conserved between monocots and dicots. Plant Physiol. 96, 775–785
Cowan, I.R. (1986) Economics of carbon fixation in higher plants. In: On the economy of plant form and function, pp. 133–170, Givnish, T.J., ed. Cambridge, Cambridge
Dietz, K.-J. (1986) an evaluation of light and CO2 limitation of leaf photosynthesis by CO2 gas-exchange analysis. Planta 167, 260–263
Downton, W.J.S., Loveys, B.R., Grant, W.J.R. (1988a) Stomatal closure fully accounts for the inhibition of photosynthesis by abscisic acid. New Phytol. 108, 263–266
Downton, W.J.S., Loveys, B.R., Grant, W.J.R. (1988b) Nonuniform stomatal closure induced by water stress causes putative non-stomatal inhibition of photosynthesis. New Phytol. 110, 503–509
Evans, J.R., Sharkey, T.D., Berry, J.A., Farquhar, G.D. (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust. J. Plant Physiol. 13, 281–292
Farquhar, G.D., von Caemmerer, S., Berry, J.A. (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90
Farquhar, G.D., O'Leary, M.H., Berry, J.A. (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol. 9, 121–137
Fluhr, R., Chua, N.H. (1987) Developmental regulation of two genes encoding ribulose-bisphosphate carboxylase small subunit in pea and transgenic petunia plants: phytochrome response and blue-light induction. Proc. Natl. Acad. Sci. USA 83, 2358–2362
Guang-yao, W., Xiao-bo, Z., Xiang-yu, W. (1987) Light regulation of the synthesis of ribulose 1,5-bisphosphate carboxylase and fructose 1,6-bisphosphatase. Acta Bot. Sin. 29, 388–396
Heber, U., Neimanis, S., Dietz, K.-J. (1988) Fractional control of photosynthesis by the QB protein, the cytochrome f/b 6complex and other components of the photosynthetic apparatus. Planta 173, 267–274
Hoagland, D.R., Arnon, D.I. (1938) The water culture method for growing plants without soil. Univ. of Cal. Exp. Sta. Circ. 347
Kaufman, L.S., Thompson, W.F., Briggs, W.R. (1984) Different red light requirements for phytochrome-induced accumulation of cab RNA and rcbS RNA. Science 226, 1447–1449
Keller, J.M., Shanklin, J., Vierstra, R.D., Hershey, H.P. (1989) Expression of a functional monocotyledonous phytochrome in transgenic tobacco. EMBO J. 8, 1005–1012
Leegood, R.C. (1985) Regulation of photosynthetic CO2-pathway enzymes by light and other factors. Photosynth. Res. 6, 247–259
Nagy, B.F., Fluhr, R., Kuhlemeier, C., Kay, S., Boutry, M., Green, P., Poulsen, C., Chua, N.-H. (1986) Cis-acting elements for selective expression of two photosynthetic genes in transgenic plants. Phil. Trans. R. Soc. London Ser. B 314, 493–500
Neuhaus, H.E., Kruckeberg, A.L., Feil, R., Stitt, M. (1989) Reduced-activity mutants of phosphoglucose isomerase in the cytosol and chloroplast of Clarkia xantiana II. Study of the mechanisms which regulate photosynthate partitioning. Planta 178, 110–122
Nobel, P.S. (1977) Internal leaf area and cellular CO2 resistance: Photosynthetic implications of variations with growth conditions and plant species. Physiol. Plant. 40, 137–144
Sage, R.F. (1990) A model describing the regulation of ribulose-1,5- bisphosphate carboxylase, electron transport, and triose phosphate use in response to light intensity and CO2 in C3 plants. Plant Physiol. 94, 1728–1734
Sasaki, T., Sakihama, T., Kamikubo, T., Shinozaki, K. (1983) Phytochrome-mediated regulation of two mRNAs, encoded by nuclei and chloroplasts of ribulose-1,5-bisphosphate carboxyl-ase/oxygenase. Eur. J. Biochem. 133, 617–620
Schmidt, G.W., Mishkind, ML. (1983) Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc. Natl. Acad. Sci. USA 80, 2632–2636
Schmidt, S., Drumm-Herrel, H., Oelmuller, R., Mohr, H. (1987) Time course of competence in phytochrome-controlled appearance of nuclear-encoded plastidic proteins and messenger RNAs. Planta 170, 400–407
Sharkey, T.D. (1988) Estimating the rate of photorespiration in leaves. Physiol. Plant. 73, 147–152
Sharkey, T.D. (1989) Evaluating the role of rubisco regulation in C3 photosynthesis. Phil. Trans. R. Soc. London Ser. B 323, 435–448
Sharkey, T.D., Seemann, J.R. (1989) Mild water stress effects on carbon-reduction-cycle intermediates, RuBP carboxylase activity, and spatial homogeneity of photosynthesis in leaves. Plant Physiol. 89, 1060–1065
Sharkey, T.D., Berry, J.A., Sage, R.F. (1988a) Regulation of photosynthetic electron-transport as determined by roomtemperature chlorophyll a fluorescence in Phaseolus vulgaris L. Planta 176, 415–424
Sharkey, T.D., Kobza, J., Seemann, J.R., Brown, R.H. (1988b) Reduced cytosolic fructose-1,6-bisphosphatase activity leads to loss of O2 sensitivity in a Flaveria linearis mutant. Plant Physiol. 86, 667–671
Sharkey, T.D., Loreto, F., Vassey, T.L. (1990) Effects of stress on photosynthesis. In: Current research in photosynthesis, pp. 549–556, Baltscheffsky, M., ed. Kluwer, Dordrecht (The Netherlands)
Sharkey, T.D., Savitch, L.V., Butz, N.D. (1991) Photometric method for routine determination of kcat and carbamylation of rubisco. Photosynth. Res., in press
Silverthorne, J., Tobin, E.M. (1984) Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc. Natl. Acad. Sci. USA 81, 1112–1116
Stiekema, W.J., Wimpee, C.F., Silverthorne, J., Tobin, E.M. (1983) Phytochrome control of the expression of two nuclear genes encoding chloroplast proteins in Lemna gibba L. G-3. Plant Physiol. 72, 717–724
Terashima, I., Wong, S.-C., Osmond, C.B., Farquhar, G.D. (1988) Characterisation of non-uniform photosynthesis induced by abscisic acid in leaves having different mesophyll anatomies. Plant Cell Environ. 29, 385–394
Valentin, K., Zetsche, K. (1989) The genes of both subunits of ribulose-1,5-bisphosphate carboxylase constitute an operon on the plastome of a red alga. Curr. Genet. 16, 203–209
Vassey, T.L. (1988) Phytochrome mediated regulation of sucrose phosphate synthase activity in maize. Plant Physiol. 88, 540–542
Vassey, T.L. (1989) Light/dark profiles of sucrose phosphate synthase, sucrose synthase, and acid invertase in leaves of sugarbeet. Plant Physiol. 89, 347–351
Vassey, T.L., Sharkey, T.D. (1989) Mild water stress of Phaseolus vulgaris plants leads to reduced starch synthesis and extractable sucrose phosphate synthase activity. Plant Physiol. 89, 1066–1070
Wehmeyer, B., Cashmore, A.R., Schafer, E. (1990) Photocontrol of the expression of genes encoding chlorophyll a/b binding proteins and small subunit of ribulose-1,5-bisphosphate carboxylase in etiolated seedlings of Lycopersicon esculentum (L.) and Nicotiana tabacum (L.). Plant Physiol. 93, 990–997
Winter, U., Feierabend, J. (1990) Multiple coordinate controls contribute to a balanced expression of ribulose-1,5-bisphosphate carboxylase/oxygenase subunits in rye leaves. Eur. J. Biochem. 187, 445–453
Wintermans, J.G.F.M., DeMots, A. (1965) Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 109, 448–453
Woodrow, I.E., Berry, J.A. (1988) Enzymatic regulation of photosynthetic CO2 fixation in C3 plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39, 533–594
Author information
Authors and Affiliations
Additional information
This research was supported by U.S. Department of Energy contracts DE-FG02-87ER60568 to T.D.S. and DE-FG02-88ER 13968 to R.D.V. We thank Drs. Joel Cherry and Howard P. Hershey for assistance with the transgenic plants.
Rights and permissions
About this article
Cite this article
Sharkey, T.D., Vassey, T.L., Vanderveer, P.J. et al. Carbon metabolism enzymes and photosynthesis in transgenic tobacco (Nicotiana tabacum L.) having excess phytochrome. Planta 185, 287–296 (1991). https://doi.org/10.1007/BF00201046
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00201046