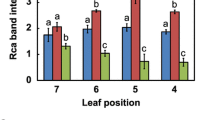
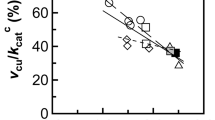
Abstract
Continuous removal of fruits from soybean plants (Glycine max [L.] Merr.) causes a redistribution of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco, EC 4.1.1.39) from the soluble to the insoluble phase of leaf extracts. The extent of this redistribution is genotype-dependent. We previously reported that insoluble Rubisco occurs in a high-molecular-mass complex together with a protein composed of 30-kDa subunits (S.J. Crafts-Brander et al., Planta, 183, 300–306). In the present study, the Rubisco Complex Protein (RCP), was isolated from the Rubisco-RCP complex by gel-filtration chromatography in 4 M urea. Under these conditions, RCP migrated with an apparent molecular mass of 120 kDa, indicating that the protein maintains a tetrameric structure even in 4 M urea. Once freed of urea, purified RCP was soluble, but formed insoluble complexes with Rubisco from soybean, tobacco and spinach when RCP and Rubisco were incubated in a ratio of 1∶1 by weight. Purified Rubisco and RCP also associated into a high-molecular-mass complex when either component was in several-fold excess, but in this case the complex was soluble. Similarly, the amount of Rubisco sequestered as an insoluble Rubisco:RCP complex in leaf extracts of different soybean genotypes was related to the relative amounts of Rubisco and RCP present in the extracts. Thus, with both purified components and in leaf extracts, formation of an insoluble complex between Rubisco and RCP required a precise stoichiometry. Antibodies directed against purified RCP detected an accumulation of RCP in soybean leaves around the time of flowering. The RCP was also detected in petioles, stems, and pod walls of soybean, but not seeds. Fruit removal caused a marked increase in the amount of RCP in the leaves to levels as high as 15% of the total soluble protein. The accumulation of RCP in response to source:sink manipulations was similar to soybean vegetative storage proteins (VSPs). However, immunogold-localization showed that RCP was located in the cytosol of leaves, compartmentalized separate from both Rubisco and the VSPs. Thus, the physiological relevance of the specific association between RCP and Rubisco is obscure.
Similar content being viewed by others
Abbreviations
- Rubisco:
-
ribulose-1,5-bisphosphate carboxylase/oxygenase (EC 4.1.1.39)
- RCP:
-
rubisco complex protein
- VSPs:
-
vegetative storage proteins
References
Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of proteindye binding. Anal. Biochem. 72, 248–259
Chua, N.-H. (1980) Electrophoretic analysis of chloroplast proteins. Methods Enzymol. 69, 434–446
Crafts-Brandner, S.J., Egli, D.B. (1987) Sink removal and leaf senescence in soybean. Cultivar effects. Plant Physiol. 85, 662–666
Crafts-Brandner, S.J., Salvucci, M.E., Egli, D.B. (1990) Changes in ribulosebisphosphate carboxylase/oxygenase and ribulose 5-phosphate kinase abundances and photosynthetic capacity during leaf senescence. Photosynth. Res. 23, 223–230
Crafts-Brandner, S.J., Salvucci, M.E., Egli, D.B. (1991) Fruit removal in soybean induces the formation of an insoluble form of ribulose-1,5-bisphosphate carboxylase/oxygenase in leaf extracts. Planta 183, 300–306
Fehr, W.R., Caviness, C.E. (1977) Stages of soybean development. Spec.Rep. No. 80, Cooperative Extension Service, Agricultural and Home Economics Experiment Station, Iowa State University, Ames, USA
Ford, D.M., Shibles, R. (1988) Photosynthesis and other traits in relation to chloroplast number during soybean leaf senescence. Plant Physiol. 86, 108–111
Franceschi, V.R., Grimes, H.D. (1991) Low levels of atmospheric methyl jasmonate induce the accumulation of soybean vegetative storage proteins and anthocyanins. Proc. Natl. Acad. Sci. USA 88, 6745–6749
Franceschi, V.R., Wittenbach, VA., Giaquinta, R.T. (1983) Paraveinal mesophyll of soybean leaves in relation to assimilate transfer and compartmentation. III. Immunohistochemical localization of specific glycopeptides in the vacuole after depodding. Plant Physiol. 72, 586–589
Iwanij, V, Chua, N.-H., Siekevitz, P. (1975) Synthesis and turnover of ribulose bisphosphate carboxylase and of its subunits during the cell cycle of Chlamydomonas reinhardtii. J. Cell Biol. 64, 572–585
Makino, A., Mae, T., Ohira, K. (1983) Photosynthesis and ribulose-1,5-bisphosphate carboxylase in rice leaves. Changes in photosynthesis and enzymes involved in carbon assimilation from leaf development through senescence. Plant Physiol. 73, 1002–1007
Salvucci, M.E., Portis, A.R., Jr., Ogren, W.L. (1986) Purification of riblose-1,5-bisphosphate carboxylase/oxygenase with high specific activity by fast protein liquid chromatography. Anal. Biochem. 153, 97–101
Staswick, P.E. (1990) Novel regulation of vegetative storage protein genes. Plant Cell 2, 1–6
Tranbarger, T.J., Franceschi, V.R., Hildebrand, D.F., Grimes, H.D. (1991) The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell 3, 973–987
Vogeli, U., Freeman, JW., Chappell, J. (1990) Purification and characterization of an inducible sesquiterpene cyclase from elicitor-treated tobacco cell suspension cultures. Plant Physiol. 92, 182–187
Wittenbach, V.A. (1979) Ribulose bisphosphate carboxylase and proteolytic activity in wheat leaves from anthesis through senescence. Plant Physiol. 64, 884–887
Wittenbach, V.A. (1982) Effect of pod removal on leaf senescence in soybeans. Plant Physiol. 70, 1544–1548
Wittenbach, V.A. (1983) Purification and characterization of a soybean leaf storage glycoprotein. Plant Physiol. 73, 125–129
Author information
Authors and Affiliations
Corresponding author
Additional information
Kentucky Agricultural Experiment Station Journal Article No. 93-3-162
We wish to acknowledge L.F. Staples and J.C. Anderson for their expert technical assistance. Electron microscopy was performed by the Nano-Probe Laboratory, Lucille Parker Markey Cancer Center, University of Kentucky. We thank Dr. K.C. Vaughn, USDA-ARS, for providing guidance pertaining to immunogold-localization procedures.
Rights and permissions
About this article
Cite this article
Crafts-Brandner, S.J., Salvucci, M.E. The Rubisco complex protein: A protein induced by fruit removal that forms a complex with ribulose-1,5-bisphosphate carboxylase/oxygenase. Planta 194, 110–116 (1994). https://doi.org/10.1007/BF00201041
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00201041