Abstract
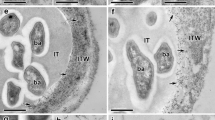
The influence exerted by Pseudomonas fluorescens, strain 63-28R, in stimulating plant defense reactions was investigated using an in-vitro system in which Ri T-DNA-transformed pea (Pisum sativum L.) roots were subsequently infected with Pythium ultimum. Cytological investigations of samples from P. fluorescens-inoculated roots revealed that the bacteria multiplied abundantly at the root surface and colonized a small number of epidermal and cortical cells. Penetration of the epidermis occurred through the openings made by the disruption of the fibrillar network at the junction of adjacent epidermal cell walls. Direct cell wall penetration was never observed and bacterial ingress into the root tissues proceeded via an intercellular route. Striking differences in the extent of fungal colonization were observed between bacterized and non-bacterized pea roots following inoculation with P. ultimum. In non-bacterized roots, the pathogen multiplied abundantly through most of the tissues while in bacterized roots, pathogen growth was restricted to the epidermis and the outer cortex. At the root surface, the bacteria interacted with the pathogen, in a way similar to that observed in dual culture tests. Most Pythium cells were severely damaged but fungal penetration by the bacteria was never observed. Droplets of the amorphous material formed upon interaction between the bacteria and the host root were frequently found at the fungal cell surface. Incubation of sections with a β-1,4-exoglucanase-gold complex revealed that the cell wall of markedly altered Pythium hyphae was structurally preserved. Successful penetration of the root epidermis was achieved by the few hyphae of P. ultimum that could escape the first defensive line in the rhizosphere. Most hyphae of the pathogen that penetrated the epidermis exhibited considerable changes. The unusual occurrence of polymorphic wall appositions along the host epidermal cells was an indication that the host plant was signalled to defend itself through the elaboration of physical barriers.
Similar content being viewed by others
Abbreviations
- AGL:
-
Aplysia gonad lectin
- PGPR:
-
plant growth-promoting rhizobacteria
References
Albert F, Anderson AJ (1987) The effect of Pseudomonas putida colonization on root surface peroxidase. Plant Physiol 85: 537–541
Alström S (1991) Induction of disease resistance in common bean susceptible to halo blight bacterial pathogen after seed bacterization with rhizosphere Pseudomonads. J Gen Appl Microbiol 37: 495–501
Bécard G, Fortin JA (1988) Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol 108: 211–218
Benhamou N (1989) Preparation and applications of lectin-gold complexes. In: Hayat MA (ed) Colloidal gold, principles, methods and applications, vol 1. Academic Press, New York, pp 95–143
Benhamou N, Chamberland H, Ouellette GB, Pauzé FJ (1987) Ultrastructural localization of β-1,4-d-glucans in two pathogenic fungi and in their host tissues by means of an exoglucanase-gold complex. Can J Microbiol 33: 405–417
Benhamou N, Gilboa-Garber N, Trudel J, Asselin A (1988) A new lectin-gold complex for the ultrastructural localization of galacturonic acids. J Histochem 36: 1403–1411
Benhamou N, Fortin JA, Hamel C, St Arnaud M, Shatilla A (1994) Resistance responses of mycorrhizal Ri T-DNA transformed carrot roots to infection by Fusarium oxysporum f. sp. chrysanthemi. Phytopathology 84: 958–968
Campbell JN, Cass DD, Peteya DJ (1987) Colonization and penetration of intact canola seedling roots by an opportunistic fluorescent Pseudomonas sp. and the response of host tissue. Phytopathology 77: 1166–1173
Chérif M, Benhamou N, Bélanger RR (1991) Ultrastructural and cytochemical studies of fungal development and host reactions in cucumber plants infected by Pythium ultimum. Physiol Mol Plant Pathol 39: 353–375
Chérif M, Benhamou N, Menzies JG, Belanger RR (1992) Silicon induced resistance in cucumber plants against Pythium ultimum. Physiol Mol Plant Pathol 41: 411–425
Chet I. (1993) Biotechnology in plant disease control. John Wiley and Sons, New York, 373 pp
Chet I, Ordentlich A, Shapira R, Oppenheim A (1990) Mechanisms of biocontrol of soil-borne plant pathogens by rhizobacteria. Plant and Soil 129: 85–92
Coulombe P, Kan FWK, Bendayan M (1988) Introduction of a high resolution cytochemical method for studying the distribution of phospholipidids in biological tissues. Eur J Cell Biol 46: 564–576
Défago G, Berling CH, Burger U, Haas D, Kahr G, Keel C, Voisard C, Wirthner P, Wüthrich B (1990) Suppression of black root rot of tobacco and other root diseases by strains of Pseudomonas fluorescens: potential applications and mechanisms. In: Hornby D (ed) Biological control of soil-borne plant pathogens. CAB Int. Wallingford, Oxon, UK, pp 93–108
Downie JA (1994) Signalling strategies for nodulation of legumes by rhizobia. Trends Microbiol 2: 318–324
Frens G (1973) Controlled nucleation for regulation of the particle size in monodisperse gold solutions. Nature (London) Phys Sci 241: 20–22
Gaastra W, de Graaf FK (1982) Host specific fimbrial adhesion of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev 46: 129–161
Gold SE, Stanghellini ME (1985) Effects of temperature on Pythium root rot of spinach grown under hydroponic conditions. Phytopathology 75: 333–337
Hajlaoui MR, Benhamou N, Bélanger RR (1992) Cytochemical study of the antagonistic activity of Sporothrix flocculosa against Erisiphe graminis var. tritici. Phytopathology 82: 583–589
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 434: 301–307
Kloepper JW (1991). Plant growth-promoting rhizobacteria as biological control agents of soilborne diseases. In: Bay-Petersen J (ed) Biological control of plant diseases (FFTC Booh series No 42), Food and Fertilizer Technology Center, Taiwan, pp 142–156
Kloepper JW (1993) Plant growth-promoting rhizobacteria as biological control agents. In: Metting B (ed) Soil microbial technologies. M. Dekker Inc., New York, pp 255–274
Kloepper JW, Schroth MN (1981) Plant growth-promoting rhizobacteria and plant growth under gnotobiotic conditions. Phytopathology 71: 642–644
Lam ST, Gaffney TD (1993) Biological activity of bacteria used in plant pathogen control. In: Chet I (ed) Biotechnology in plant disease control. John Wiley & Sons, New York, pp 139–156
Lemanceau P (1989) Role of competition for carbon and iron in mechanisms of suppressiveness to Fusarium wilts. In: Tjamos EC, Beckman C (eds) Vascular wilts diseases of plants. SpringerVerlag, Heidelberg, Berlin, pp 385–396
Lemanceau P, Alabouvette C (1993) Suppression of Fusarium wilts by fluorescent Pseudomonads: mechanisms and applications. Biocontrol Sci Technol 3: 219–234
Mauch F, Staehelin LA (1989) Functional implications of the subcellular localization of ethylene-induced chitinase and β-1,3-glucanase in bean leaves. Plant Cell 1: 447–457
Mazzucchi U (1983) Recognition of bacteria by plants. In: Callow JA (ed) Biochemical plant pathology. John Wiley & Sons Ltd, New York, pp 299–322
O'Sullivan DJ, O'Gara F (1992) Traits of fluorescent Pseudomonas spp.involved in suppression of plant root pathogens. Microbiol Rev 56: 662–676
Paulitz TC, Zhou T, Rankin L (1992) Selection of rhizosphere bacteria for biological control of Pythium aphanidermatum on hydroponically grown cucumber. Biol Control 2: 226–237
Rankin L, Paulitz TC (1994) Evaluation of rhizosphere bacteria for biological control of Pythium root rot of greenhouse cucumbers in hydroponic culture. Plant Dis 78: 447–451
Scheffer RJ (1983) Biological control of dutch elm disease by Pseudomonas species. Ann Appl Biol 103: 21–30
Scher FM, Kloepper JW, Singleton CA (1985) Chemotaxis of fluorescent Pseudomonas spp. to soybean seed exudates in vitro and in soil. Can J Microbiol 31: 570–574.
Schroth MN, Becker JO (1990) Concepts of ecological and physiological activities of rhizobacteria related to biological control and plant growth promotion. In: Hornby D (ed) Biological control of soil-borne plant pathogens. CAB Int Wallingford, Oxon, UK, pp 389–414
van Peer R, Niemann GJ, Schippers B (1991) Induced resistance and phytoalexin accumulation in biological control of Fusarium wilt of carnation by Pseudomonas sp. strain WCS417r. Phytopathology 81: 728–734
Weller DM (1988) Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol 26: 379–407
Wiehe W, Hecht-Buchholtz Ch, Höflich G (1994) Electron microscopic investigations on root colonization of Lupinus albus and Pisum sativum with two associative plant growth-promoting rhizobacteria, Pseudomonas fluorescens and Rhizobium leguminosarum bv. trifolii. Symbiosis 17: 15–31
Zdor RE, Anderson AJ (1992) Influence of root colonizing bacteria on the defense responses of bean. Plant and Soil 140: 99–107
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors wish to thank Sylvain Noël for excellent technical assistance. This study was supported by grants from the Fonds Québécois pour la formation de chercheurs et l'Aide à la Recherche (FCAR), the Natural Sciences and Engineering Council of Canada (NSERC) and the Ministère de l'Industrie, du Commerce, de la Science et de la Technologie (SYNERGIE).
Rights and permissions
About this article
Cite this article
Benhamou, N., Bélanger, R.R. & Paulitz, T.C. Pre-inoculation of Ri T-DNA-transformed pea roots with Pseudomonas fluorescens inhibits colonization by Pythium ultimum Trow: an ultrastructural and cytochemical study. Planta 199, 105–117 (1996). https://doi.org/10.1007/BF00196887
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00196887