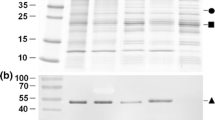
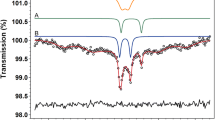
Abstract
The uptake of 59Fe from FeCl3, ferric (Fe3+) citrate (FeCitr) and Fe3+-EDTA (FeEDTA) was studied in leaf mesophyll of Vigna unguiculata (L.) Walp. Uptake rates decreased in the order FeCl3>FeCitr≫FeEDTA, and uptake depended on an obligatory reduction step of Fe3+ to Fe2+, after which the ion could be taken up independently of the chelator, citrate. Uptake was strongly increased by photosynthetically active light (λ>630 nm), and kinetic analysis revealed saturation kinetics with a K m (FeCitr) of 80–110 μM. In the presence of an external Fe2+ scavenger, bathophenanthroline disulfonate, the mesophyll also reduced external FeCitr with a K m of approx. 50–60 μM. The reduction rates for FeCitr were five-to eightfold higher than necessary for uptake. Purified plasma membranes from leaves revealed an NADH-dependent FeCitr- and FeEDTA-reductase activity, which had a pH optimum of 6.5–6.8 and a K m of approx. 20 μM for NADH. Under anaerobic conditions, a K m of 130–170 μM for ferric chelates was obtained, while in the presence of oxygen a K m (FeCitr) of approx. 100 μM was found. It is concluded that the leaf plasma membrane provides a ferric-chelate-reductase activity, which plays a crucial role in iron uptake of leaf cells. Under in-vivo conditions, however, reactive oxygen species or strong (blue) light may also contribute to the obligatory reduction of Fe3+ prior to uptake.
Similar content being viewed by others
Abbreviations
- BPDS:
-
bathophenanthroline disulfonate
- DCMU:
-
3-(3,4 dichlorophenyl)-1,1-dimethyl urea
- FCR:
-
ferricchelate reductase
- FeCitr:
-
Fe3+-citrate
- FeEDTA:
-
Fe3+-EDTA
- PM:
-
plasma membrane
References
Allnutt, F.C.T., Bonner, W.D. Jr. (1987) Evaluation of reductive release as a mechanism for iron uptake from Ferrioxamine B by Chlorella vulgaris. Plant Physiol. 85, 751–756
Barr, R., Sandelius, A.S., Crane, F.L., Morre D.J. (1986) Redox reactions in tonoplast and plasma membrane isolated from soybean hypocotyls by free-flow electrophoresis. Biochim. Biophys. Acta 852, 254–261
Bezkorovainy, A. (1980) Biochemistry of nonheme iron, pp. 1–24, Plenum Press, New York
Bienfait, H.F. (1988) The Turbo reductase in plant plasma membranes. In: Plasma membrane oxidoreductases in control of animal and plant growth, pp. 89–98, Crane, F.L., Morre, D.J., Löw, H.E., eds. Plenum Press, New York
Bienfait, H.F., van den Briel, W., Meslund-Mul, T. (1985) Free space iron pools in roots. Plant Physiol. 78, 596–600
Böttger, M., Crane, F.L., Barr, R. (1991) Physiological aspects of transplasma membrane electron transport in roots and cultured carrot tissue. In: Oxidoreduction at the plasma membrane: Relation to growth and transport, vol. II., pp. 207–236, Crane, F.L., Morre, D.J., Löw, H.E., eds. CRC Press Inc., Boca Raton
Brown, J.C., Jolley, V.D. (1986) An evaluation of concepts related to iron deficiency chlorosis. J Plant Nutr. 9, 175–182
Brüggemann, W., Janiesch, P. (1987) Characterization of plasma membrane H+-ATPase from salt-tolerant and salt-sensitive Plantago species. J. Plant Physiol. 130, 395–411
Brüggemann, W., Janiesch, P. (1989) Comparison of plasma membrane ATPase from salt-treated and salt-free grown Plantago maritima L. J. Plant Physiol. 134, 20–25
Brüggemann, W., Moog, P.R. (1989) NADH-dependent Fe3+- EDTA and oxygen reduction by plasma membrane vesicles from barley roots. Physiol. Plant. 75, 245–254
Brüggemann, W., Moog, P.R., Nakagawa, H., Janiesch, P., Kuiper, P.J.C. (1990). Plasma membrane-bound Fe3+-EDTA reductase and iron deficiency in tomato (Lycopersicon esculentum). Is there a Turbo reductase? Physiol. Plant. 79, 339–346
Cakmak, I., van de Wetering, D.A.M., Marschner, H., Bienfait, H.F. (1987) Involvement of superoxide radical in extracellular ferric reduction by iron-deficient bean roots. Plant Physiol. 85, 310–314
Chaney, R.L., Brown, J.D., Tiffin, L.O. (1972) Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol. 50, 208–213
Dhamawardhane, S., Stern, A.I., Rubinstein, B. (1987) Light-stimulated transplasmalemma electron transport in oat mesophyll cells. Plant Sci. 51, 193–201
Gautier, H., Vavasseur, A., Lasceve, G., Bourdet, A.M. (1992) Redox processes in the blue light response of guard cell protoplasts of Commelina communis L. Plant Physiol. 98, 34–38
Hunte, C., Schnabl, H., Traub, O., Willecke, K., Schulz, M. (1992) Immunological evidence of connexin-like protein in the plasma membrane of Vicia faba L. Bot. Acta 105, 104–110
Kjellbohm, P., Larsson, C. (1984) Preparation and polypeptide composition of chlorophyll-free plasma membranes from leaves of light-grown spinach and barley. Physiol. Plant. 62, 501–509
Lass, B., Thiel, G., Ullrich-Eberius, C.I. (1986) Electron transport across the plasmalemma of Lemna gibba G1. Planta 169, 251–259
Lundborg, T., Widell, S., Larsson, C. (1981) Distribution of ATPases in wheat root membranes separated by phase partitioning. Physiol. Plant. 52, 89–95
Macri, F., Braidot, E., Petrussa, E., Zancani, M., Vianello, A. (1992) Ferric ion and oxygen reduction at the surface of protoplasts and cells of Acer pseudoplatanus. Bot. Acta 105, 97–103
Marschner, H., Römheld, V., Knissel, M. (1986) Different strategies in higher plants in mobilisation and uptake of iron. J. Plant Nutr. 9, 695–713
Römheld, V., Marschner, H. (1983) Mechanism of iron uptake by peanut plants. I. Fe(III) reduction, chelate splitting and release of phenolics. Plant Physiol. 71, 949–954
Schmidt, W., Janiesch, P., Brüggemann, W. (1990) FeEDTA reduction in roots of Plantago lanceolata by a NADH-dependent plasma membrane-bound redox system. J. Plant Physiol. 136, 51–55
Terry, N., Abadia, J. (1986) Function of iron in chloroplasts. J. Plant Nutr. 9, 609–646
Author information
Authors and Affiliations
Additional information
This work was supported by the SCIENCE program of the European Community (contract no. SC1000344; P.R.M.). We wish to thank P. Siersma and C. Winter for their cooperation at the Central Isotope Laboratory of the Biological Centre of the University of Groningen.
Rights and permissions
About this article
Cite this article
Brüggemann, W., Maas-Kantel, K. & Moog, P.R. Iron uptake by leaf mesophyll cells: The role of the plasma membrane-bound ferric-chelate reductase. Planta 190, 151–155 (1993). https://doi.org/10.1007/BF00196606
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00196606