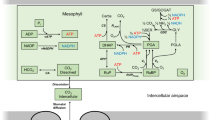
Abstract
A model of the C 3 photosynthetic system is developed which describes the sensitivity of the steadystate rate of carbon dioxide assimilation to changes in the activity of several enzymes of the system. The model requires measurements of the steady-state rate of carbon dioxide assimilation, the concentrations of several intermediates in the photosynthetic system, and the concentration of the active site of ribulose 1,5-bisphosphate carboxyalse/oxygenase (Rubisco). It is shown that in sunflowers (Helianthus annuus L.) at photon flux densities that are largely saturating for the rate of photosynthesis, the steady-stete rate of carbon dioxide assimilation is most sensitive to Rubisco activity and, to a lesser degree, to the activities of the stromal fructose, 6-bisphosphatase and the enzymes catalysing sucrose synthesis. The activities of sedoheptulose 1,7-bisphosphatase, ribulose 5-phosphate kinase, ATP synthase and the ADP-glucose pyrophosphorylase are calculated to have a negligible effect on the flux under the high-light conditions. The utility of this analysis in developing simpler models of photosynthesis is also discussed.
Similar content being viewed by others
Abbreviations
- c i :
-
intercellular CO2 concentration
- C supJinfP :
-
control coefficient for enzyme P with respect to flux J
- DHAP:
-
dihydroxyacetonephosphate
- E4P:
-
erythrose 4-phosphate
- F6P:
-
fructose 6-phosphate
- FBP:
-
fructose 1,6-bisphosphate
- FBPase:
-
fructose 1,6-bisphosphatase
- G3P:
-
glyceraldehyde 3-phosphate
- G1P:
-
glucose 1-phosphate
- G6P:
-
glucose 6-phosphate
- Pi:
-
inorganic phosphate
- PCR:
-
photosynthetic carbon reduction
- PGA:
-
3-phosphoglyceric acid
- PPFD:
-
photosynthetically active photon flux density
- R Jn :
-
response coefficient for effector n with respect to flux J
- R5P:
-
ribose 5-phosphate
- Rubisco:
-
ribulose 1,5-bisphosphate carboxylase/oxygenase
- Ru5P:
-
ribulose 5-phosphate
- RuBP:
-
ribulose 1,5-bisphosphate
- S7P:
-
sedoheptulose 7-phosphate
- SBP:
-
sedoheptulose 1,7-bisphosphate
- SBPase:
-
sedoheptulose 1,7-bisphosphatase
- SPS:
-
sucrose-phosphate synthase
- Xu5P:
-
xylulose 5-phosphate
- ɛ P n :
-
elasticity coefficient for effector n with respect to the catalytic velocity of enzyme P
References
Andrews, T.J., Lorimer, G.H. (1987) Rubisco: structure, mechanisms, and prospects for improvement. In: The biochemistry of plants, vol. 10, pp. 131–218, Hatch, M.D., ed. Academic Press, New York
Ashton, A.R. (1982) A role for ribulose 1,5-bisphosphate carboxylase as a metabolite buffer. FEBS Lett. 145, 1–7
Badger, M.R., Sharkey, T.D., von Caemmerer, S. (1984) The relationship between steady-state gas exchange of bean leaves and the levels of carbon reduction cycle intermediates. Planta 160, 305–313
Bassham, J.A., Krause, G.H. (1969) Free energy changes and metabolic regulation in steady-state photosynthetic carbon reduction. Biochim. Biophys. Acta 189, 207–221
Cardon, Z.G., Mott, K.A. (1989) Evidence that ribulose 1,5-bisphosphate binds to inactive sites of RuBP carboxylase in vivo and an estimate for the rate constant for dissociation. Plant Physiol. 89, 1253–1257
Charles, S.A., Halliwell, B. (1980) Properties of freshly purified and thiol treated chloroplast fructose bisphosphatase. Biochem. J. 185, 689–693
Copeland, L., Preiss, J. (1981) Purification of the spinach leaf ADP-glucose pyrophosphorylase. Plant Physiol. 68, 996–1001
Evans, J.R., Sharkey, T.D., Berry, J.A., Farquhar, G.D. (1986) Carbon isotope discrimination measured concurrently with gasexchange to investigate CO2 diffusion in leaves of higher plants. Aust. J. Plant Physiol. 13, 281–292
Farquhar, G.D. (1979) Models describing the kinetics of ribulose bisphosphate carboxylase/oxygenase. Arch. Biochem. Biophys. 193, 456–468
Farquhar, G.D., von Caemmerer, S., Berry, J.A. (1980) A biochemical model of photosynthetic CO2 fixation in leaves of C3 species. Planta 149, 78–90
Furbank, R.T., Foyer, C.H., Walker, D.A. (1987) Interactions between ribulose 1,5-bisphosphate carboxylase and stromal metabolites. Biochim. Biophys. Acta 894, 165–173
Gardemann, A., Stitt, M., Heldt, H.W. (1983) Control of CO2 fixation. Regulation of spinach ribulose 5-phosphate kinase by stromal metabolites. Biochim. Biophys. Acta 722, 51–60
Heber, U., Neimanis, S., Deitz, K.J., Viil, J. (1986) Assimilatory power as a driving force in photosynthesis. Biochim. Biophys. Acta 852, 144–155
Jordan, D.B., Ogren, W.L. (1984) The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase. Plant 161, 308–313
Kacser, H., Burns, J.A. (1973) The control of flux. Symp. Soc. Exp. Biol. 27, 65–104
Laing, W.A., Stitt, M., Heldt, H.W. (1981) Changes in the activity of ribulose-phosphate kinase and fructose and sedoheptulosebisphosphatase in chloroplasts. Biochim. Biophys. Acta 637, 348–359
Leegood, R.C., Walker, D.A. (1982) Regulation of fructose 1,6-bisphosphatase activity in leaves. Planta 156, 449–456
Leegood, R.C., Foyer, C.H., Walker, D.A. (1985) Regulation of the Bensen-Calvin Cycle. In: Photosynthetic mechanisms and the environment, pp. 191–258, Barber, J., Baker, N., eds. Elsevier, New York
Lowry, O.H., Passoneau, J.U. (1972) A flexible system of enzymic analysis. Academic Press, New York London
Mills, J.D., Mitchell, P. (1982) Thiol modulation of CF0-CF1 stimulates acid-base dependent phosphorylation of ADP by broken pea chloroplasts. FEBS Lett. 144, 63–67
Mott, K.A. (1989) Do stomata respond to CO2 concentrations other than intercellular? Plant Physiol. 86, 200–203
Neuhaus, H.E., Kruckeberg, A.L., Feil, R., Stitt, M. (1989) Decreased-activity mutants of phosphoglucoisomerase in the chloroplast and cytosol of Clarkia xantiana. Planta 178, 110–122
Perchorovicz, J.T., Raynes, D.A., Jensen, R.G. (1981) Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc. Natl. Acad. Sci. USA 78, 2985–2989
Preiss, J. (1982) Regulation of the biosynthesis and degradation of starch. Annu. Rev. Plant Physiol. 33, 431–454
Quick, W.P., Schurr, U., Scheibe, R., Schulze, E.-D., Rodermel, S.R., Bogorad, L., Stitt, M. (1991) Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in tobacco transformed with ‘antisense’ rbcS. Impact on photosynthesis in ambient growth conditions. Planta 183, 542–544
Soulie, J.M., Buc, J., Meunier, J.-C., Pradel, J., Ricard, J. (1981) Molecular properties of chloroplastic thioredoxin-f and the regulation of fructose 1,6-bisphosphatase. Eur. J. Biochem. 119, 497–502
Stitt, M. (1989) Control analysis of photosynthetic sucrose synthesis: assignment of elasticity coefficients and flux control coefficients to the cytosolic fructose 1,6-bisphosphatase and sucrose phosphate synthase. Philos. Trans. R. Soc. London, Ser. B323, 327–338
Stitt, M. (1991) Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Environ. 14, 741–762
Stitt, M., Quick, W.P., Schurr, U., Rodermel, S.R., Bogorad, L. (1991) Decreased ribulose-1,5-bisphosphate carboxylase-oxygenause in tobacco transformed with ‘antisense’ rbcS. II. Fluxcontrol coefficients for photosynthesis in varying light, CO2 and air humidity. Planta 183, 555–566
Stitt, M., Wirtz, W., Heldt, H.W. (1980) Metabolite levels during induction in the chloroplast and extrachloroplast compartments of spinach protoplasts. Biochim. Biophys. Acta 593, 85–102
von Caemmerer, S., Farquhar, G.D. (1985) Kinetics and activation of Rubisco and some preliminary modelling of RuP2 pool sizes. In: Kinetics of photosynthesis and photorespiration in C3 plants, vol. 1, pp. 46–48, Viil, J., ed., Tallinn, Valgus
von Caemmerer, S., Edmondson, D.L. (1986) Relationship between steady-state gas exchange, in vivo ribulose bisphosphate carboxylase activity and some carbon reduction cycle intermediates in Raphanus sativus. Aust. J. Plant Physiol. 13, 669–688
Woodrow, I.E. (1986) Control of the rate of photosynthetic carbon dioxide fixation. Biochim. Biophys. Acta 851, 181–192
Woodrow, I.E., Berry, J.A. (1988) Enzymatic regulation of photosynthetic CO2 fixation in C3 plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39, 533–94
Woodrow, I.E., Mott, K.A. (1988) A quantitative assessment of the degree to which RuBP carboxylase/oxygenase limits the steady-state rate of photosynthesis during sun-shade acclimation in Helianthus annuus. Aust. J. Plant Physiol. 15, 253–262
Woodrow, I.E., Walker, D.A. (1980) Light-mediated activation of stromal sedoheptulose bisphosphatase. Biochem. J. 191, 845–849
Woodrow, I.E., Walker, D.A. (1983) Regulation of stromal sedoheptulose 1,7-bisphosphatase activity and its role in controlling the reductive pentose phosphate pathway. Biochim. Biophys. Acta 722, 508–516
Woodrow, I.E., Murphy, D.J., Walker, D.A. (1983) Regulation of photosynthetic carbon metabolism. The effect of inorganic phosphate on stromal sedoheptulose 1,7-bisphosphatase. Eur. J. Biochem. 132, 121–123
Woodrow, I.E., Ball, J.T., Berry, J.A. (1986) A general expression for the control of the rate of photosynthetic CO2 fixation by stomata, the boundary layer and radiation exchange. Progr. Photosynth. Res. 4, 225–228
Woodrow, I.E., Ball, J.T., Berry, J.A. (1990) Control of photosynthetic carbon dioxide fixation by the boundary layer, stomata and ribulose 1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 13, 339–347
Author information
Authors and Affiliations
Additional information
This research was funded by an Australian Research Council grant to I.E.W. and was undertaken during a visity by K.A.M. to the James Cook University of North Queensland. The expert help of Glenys Hanley and Mick Kelly is greatly appreciated.
Rights and permissions
About this article
Cite this article
Woodrow, I.E., Mott, K.A. Modelling C3 photosynthesis: A sensitivity analysis of the photosynthetic carbon-reduction cycle. Planta 191, 421–432 (1993). https://doi.org/10.1007/BF00195743
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00195743