Abstract
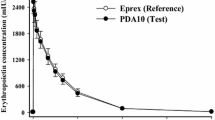
The pharmacokinetics of recombinant human erythropoietin (RhEPO) were investigated after subcutaneous (s.c.) injection in the thigh and in the abdominal wall. Eleven healthy subjects, age 24.4 years (median), were studied. Each subject received two s.c. injections of 100 U·kg-1 RhEPO dissolved in 1 ml water: one injection in the thigh and another in the abdomen. Serum erythropoietin was measured regularly by radioimmunoassay until 144 h after each injection. The mean residence time was significantly longer after injection in the thigh than in the abdomen (32.7 vs 26.2 h). The estimated half-life of absorption was significantly longer after injection in the thigh than after abdominal application (14.9 vs 12.3 h). The estimated half-life of elimination was not significantly different (4.4 vs 4.8 h). The relative difference in the area under the curve between injection in the abdomen and the thigh in the same subject ranged from -36% to +68% but there was no significant difference in bioavailability. The peak concentration was not significantly different and appeared at around 10 h (Cmax thigh, 175 U·l-1 vs Cmax abdomen, 216 U·l-1). A twin-peak configuration of the concentration vs time curve with a significant second peak at 24 h was found after injection in the thigh but not after abdominal injection. In conclusion, the mean residence time was longer after administration in the thigh, probably due to delayed absorption, but bioavailability was not significantly different. Following injection in the thigh the concentration curve had two peaks. The differences may be due to regional variations in lymph flow and to physical activity. The overall differences in pharmacokinetics appeared to be too small to recommend a general preference of the injection site.
Similar content being viewed by others
References
Annable L, Cotes PM, Musset MV (1972) The second international preparation of erythropoietin, human, urinary for bioassay. Bull World Health Organ 47: 99–112
Besshyah SA, Anyaoku V, Niththyananthan R, Sharp P, Johnston DG (1991) The effect of subcutaneous injection site on absorption af human growth hormone: abdomen versus thigh. Clin Endocrinol (Oxf) 35: 409–412
Binder C (1969) Absorption of injected insulin. A clinico-pharmacological study. Muncksgaard, Copenhagen
Boelaert JR, Schurgers ML, Matthys EG, et al (1989) Comparative pharmacokinetics of recombinant erythropoietin administered by the intravenous, subcutaneous, and intraperitoneal routes in continuos ampulatory peritoneal dialysis (CAPD) patients. Perit Dial Int 9: 95–98
Bommer J, Barth HP, Zeier M, et al (1991) Efficacy comparison of intravenous and subcutaneous recombinant human erythropoietin administration in hemodialysis patients. Contrib Nephrol 88: 136–143
Brockmöller J, Köchling J, Weber W, Looby M, Roots I, Neumayer HH (1992) The pharmacokinetics and pharmacodynamics of recombinant human erythropoietin in haemodialysis patients. Br J Clin Pharmacol 34: 499–508
Eschbach JW, Kelly MR, Haley NR, Abels RI, Adamson JW (1989) Treatment of the anemia of progressive renal failure wwith recombinant human erythropoietin. N Engl J Med 321: 158–163
Evans JHC, Brocklebank JT, Bowmer CJ, Ng PC (1991) Pharmacokinetics of recombinant human erythropoietin in children with renal failure. Nephrol Dial Transplant 6: 709–714
Fernquist E, Linde B, Ostman J, Gunnarson R (1986) Effects of physical exercise on insulin absorption in insulin-dependent diabetics: a comparison between human and porcine insulin. Clin Physiol 6: 489–498
Halstenson CE, Macres M, Katz SA, et al (1991) Comparative pharmacokinetics and pharmadynamics of epoetin alfa and epoetin beta. Clin Pharmacol Ther 50: 702–712
Hildebrandt P (1991) Skinfold thickness, local subcutaneous blood flow and insulin absorption in diabetic patients. Acta Physiol Scand [Suppl] 603: 41–45
Hughes RT, Cotes PM, Oliver DO, et al (1989) Correction of the anaemia of chronic renal failure with erythropoietin: pharmacokinetic studies in patients on haemodialysis and CAPD. Contrib Nephrol 76: 122–130
Kahn RC, Shechter Y (1990) Insulin, oral hypoglycemic agents, and the pharmacology of the endocrine pancreas. In: Gilman AG, Rall TW, Nies AS, Tayler P (ed) The pharmacological basis of therapeutics, 8th edn. Pergamon Press, New York, pp 1463–1465
Kampf D, Kahl A, Passlick J et al (1989) Single-dose kinetics of recombinant human erythropoietin after intravenous, subcutaneous and intraperitoneal administration. Contrib Nephrol 76: 106–111
Kampf D, Eckardt KU, Schmalisch C, Ehmer B, Schostak M (1992) Pharmacokinetics of recombinant human erythropoietin in dialysis patients after single and multiple subcutaneous administration. Nephron 61: 393–398
Macdougall IC, Roberts DE, Neubert P, Dharmasena AD, Coles GA, Williams JDD (1989) Pharmacokinetics of recombinant human erythropoietin in patients on continuous ambulatory peritoneal dialysis. Lancet II: 425–427
Macdougall IC, Jones JM, Robinson MI, Miles JB, Coles GA, Williams JD (1991a) Subcutaneous erythropoietin therapy: comparison of three different sites of injection. Contrib Nephrol 88: 152–156
Macdougall IC, Roberts DE, Coles GA, Williams JD (1991b) Clinical pharmacokinetics of epoetin (recombinant human erythropoietin). Clin Pharmacokinet 20: 99–113
McMahon FG, Vargas R, Ryan M et al (1990) Pharmacokinetics and effects of recombinant human erythropoietin after intravenous and subcutaneous injections in healthy volunteers. Blood 76: 1718–1722
Neumayer HH, Brockmöller J, Emmanuel F, Roots I, Scigalla P, Wattenberg M (1989) Pharmacokinetics of recombinant human erythropoietin after s.c. administration and long-term i.v. treatment in patients on maintenance hemodialysis. Contrib Nephrol 76: 131–142
Nielsen OJ (1990) Pharmacokinetics of recombinant human erythropoietin in chronic haemodialysis patients. Pharmacol Toxicol 66: 83–86
Nosadini R, Kreutzenberg S, Duner E et al (1988) Porcine and human insulin absorption from subcutaneous tissues in normal and insulin-dependent diabetic subjects: a deconvolution-based approach. J Clin Endocrinol Metab 67: 551–559
Paulsen EP, Courtney JW, Duckworth WC (1979) Insulin resistance caused by massive degradation of subcutaneous insulin. Diabetes 28: 640–645
Salmonsen T, Danielson BG, Wikström B (1990) The pharmacokinetics of recombinant erythropoietin after intravenous and subcutaneous administration to healthy subjects. Br J Clin Pharmacol 29: 709–713
Salmonson T, Danielson BG, Grahnen A, Wikström B (1990) Pharmacokinetics of intravenous recombinant human erythropoietin in patients with chronic renal failure. J Int Med 228: 53–57
Schmid-Schönbein GW (1990) Microlymphatics and lymph flow. Physiol Rew 70: 987–1028
Stockenhuber F, Loibl U, Gottsauner-Wolf M et al (1991) Pharmacokinetics and dose response after intravenous and subcutaneous administration of recombinant erythropoietin in patients on regular haemodialysis treatment or continuous ambulatory peritoneal dialysis. Nephron 59: 399–402
Supersaxo A, Hein WR, Steffen H (1990) Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res 7: 167–169
Süsstrunk H, Morell B, Ziegler WH, Froesch ER (1982) Insulin absorption from the abdomen and the thigh in healthy subjects during rest and exercise: blood glucose, plasma insulin, growth hormone, adrenaline and noradrenaline levels. Diabetologia 22: 171–174
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jensen, J.D., Jensen, L.W. & Madsen, J.K. The pharmacokinetics of recombinant human erythropoietin after subcutaneous injection at different sites. Eur J Clin Pharmacol 46, 333–337 (1994). https://doi.org/10.1007/BF00194401
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00194401