Abstract
The pharmacokinetics and haemodynamic effects of isosorbide dinitrate (ISDN) have been investigated following administration of single doses as a sublingual (SL) spray (2.5 mg), sublingual tablet (5 mg) and peroral tablet (10 mg) in a randomised, placebo-controlled double-blind cross-over trial in 16 healthy volunteers.
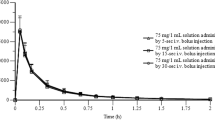
After the sublingual spray Cmax was higher (39.0 ng·ml-1) and tmax was shorter (3.9 min) than after the sublingual (22.8 ng·ml-1 and 13.8 min) and peroral (16.9 ng·ml-1 and 25.6 min) tablets. The AUC of ISDN did not differ following any of the three formulations (1031; 879; 997 ng·ml-1·min, for the spray, SL tablet and PO-tablet, respectively). Mononitrate metabolites of ISDN (IS-2-MN and IS-5-MN) and total nitrates in plasma increased in proportion to the administered dose. This indicates that the fraction of the dose absorbed was the same for all the formulations but that the extent of first-pass metabolism increased in the order sublingual spray < sublingual tablet < peroral tablet. Thus, compared to the spray, the relative bioavailability of ISDN was 48% and 28% from the sublingual and peroral tablets, respectively.
The haemodynamic effects were quantified using the a/b ratio of the finger pulse wave and the systolic blood pressure and heart rate under orthostatic conditions. For the a/b ratio of the finger pulse, the maximal effect was higher (emax=130%) and the time to emax (temax) shorter (16.6 min) after the spray than the sublingual tablet (84.4% and 25.5 min) or peroral tablet (90.2 and 31.3 min). The onset of effect was within 3, 5 and 7.5 min after the spray, sublingual and peroral tablets, respectively. A larger change in the orthostatically-induced decrease in systolic blood pressure and increase in heart rate was obtained following peroral than sublingual administration despite the similar plasma concentrations of ISDN. This probably reflects the larger amount of pharmacodynamically active mononitrate metabolites formed after oral dosing. The integrated effect following administration of 2.5 mg ISDN as spray was similar to that of a sublingual tablet of 5 mg.
Similar content being viewed by others
References
Abshagen U, Betzien G, Enderle R, Kaufmann B, Neugebauer G (1985) Pharmacokinetics and metabolism of isosorbide-dinitrate after intravenous and oral administration. Eur J Clin Pharmacol 27: 637
Ahlner J, Adersson RG, Torfgard K, Axelsson KL (1991) Organic nitrate esters: clinical use and mechanisms of actions. Pharmacol Rev 43: 353–423
Bassenge E, Holtz J, Kinadeter H, Kolin A (1981) Threshold dosages of nitroglycerin for coronary artery dilation, afterload reduction and venous pooling in conscious dogs. In: Lichtlen PR, Engel HJ, Schrey A, Swan HJC (eds) Nitrates, vol III. Springer, Berlin Heidelberg New York, pp 238–250
Bogaert MG, Rosseel MT (1983) Fate of orally given isosorbide dinitrate in man: factors of variability. Z Kardiol 72 [Suppl 3]: 11–13
Buschmann M, Wiegand A, Schnellbacher K, Bonn R, Rehe A, Trenk D, Jähnchen E, Roskamm H (1993) Comparison of the effect of two different galenic preparations of glyceryl trinitrate on pulmonary artery pressure and on finger pulse curve. Eur J Clin Pharmacol 44: 451–456
Chasseaud LF, Darragh A, Doyle E, Lambe RF, Taylor T (1984) Isosorbide dinitrate plasma concentrations and bioavailability in human subjects after administration of standard oral and sublingual formulation. J Pharm Sci 73: 699
Fung HL, Sutton SC, Kamiya A (1984) Blood vessel uptake and metabolism of organic nitrates in the rat. J Pharmacol Exp Ther 228: 334
Gascho JA, Fanelli C, Sumner A, Zelis R (1989) Effects of posture on the venodilatory response to nitroglycerin. J Appl Physiol 66: 2585–2588
Gerson JI, Allen FB, Seitzer JL, Parker FB, Markowitz AH (1982) Arterial and venous dilation by nitroprusside and nitroglycerin — is there a difference? Anesth Analg (Cleve) 61: 256–260
Gwilt DJ, Petri M, Reid DS (1983) Intravenous isosorbide dinitrate in acute left ventricular failure — a dose-response study. Eur Heart J 4: 712–717
Imhof PR, Ott B, Frankhauser P, Chu LC, Hodler J (1980) Difference in nitroglycerin dose-response in the venous and arterial beds. Eur J Clin Pharmacol 18: 455–460
Jaeger H, Lutz D, Michaelis K, Salama ZB (1987) Determination of nitrates in plasma. Drugs 33 [Suppl 4]: 9–22
Mackenzie JE, Parratt JR (1977) Comparative effects of glyceryl trinitrate on venous and arterial smooth muscle in vitro: relevance to antianginal activity. Br J Pharmacol 29: 155–160
Miller RR, Vismara LA, Williams DO, Amsterdem EA, Mason DT (1976) Pharmacological mechanisms for left ventricular unloading in clinical congestive heart failure. Differential effects of nitroprusside, phentolamine, and nitroglycerin on cardiac function and peripheral circulation. Circ Res 39: 127–133
Morrison RA, Wiegand UW, Jähnchen E, Höhmann D, Bechtold H, Meinertz T, Fung H-L (1983a) Isosorbide dinitrate kinetics and dynamics after intravenous, sublingual, and percutaneous dosing in angina. Clin Pharmacol Ther 33: 747–756
Morrison RA, Wiegand UW, Jähnchen E, Höhmann D, Kasper W, Meinertz T, Fung H-L (1983b) Hepatic extraction of isosorbide dinitrate in cardiac patients. Clin Pharmacol Ther 34: 724–731
Platzer R, Reutemann G, Galeazzi RL (1982) Pharmacokinetic of intravenous isosorbide dinitrate. J Pharmacokin Biopharm 10: 575–585
Posadas del Rio FA, Jaramillo Juárez F, Camacho Garcia R (1987) Biotransformation of organic nitrate esters in vitro by human liver kidney, intestine, and blood serum. Drug Metab Disp 16: 477–481
Rösen R, König E, Klaus W (1987) Different sensitivities of arteries and veins to glyceryltrinitrate-induced relaxation and tolerance: an “in vitro” study on isolated vessels in rabbits. Arch Int Pharmacodyn Ther 285: 226–237
Straehl P, Galeazzi RL (1985) Isosorbide dinitrate bioavailability, kinetics and metabolism. Clin Pharmacol Ther 38: 140–149
Taylor T, Chasseaud LF, Doyle E, Bonn R, Darragh A, Lambe RF (1982) Isosorbide dinitrate pharmacokinetics. Arzneimittelforschung (Drug Res) 32: 1329–1333
Taylor T, Taylor IW, Chasseaud LF, Bonn R (1987) Pharmacokinetics and metabolism of organic nitrate vasodilators, In: Bridges JW, Chasseaud LF, Gibson GG (eds) Progress in drug metabolism, vol. 10. Taylor & Francis, London New York Philadelphia, pp 207–321
Toyoda J, Hisayama T, Takayanagi I (1986) Nitro compounds (isosorbide dinitrate, 5-isosorbide mononitrate and glyceryl trinitrate) on the femoral vein and femoral artery. Gen Pharmacol 17: 89–91
Wagner F, Siefert F, Trenk D, Jähnchen E (1990) Relationship between pharmacokinetics and hemodynamic tolerance to isosorbide-5-mononitrate. Eur J Clin Pharmacol 38 [Suppl 1]: 53–59
Wiegand A, Bonn R, Wagner F, Trenk D, Jähnchen E (1991) Haemodynamic effects of glyceryl trinitrate following repeated application of a transdermal delivery system with a phasic release profile. Eur J Clin Pharmacol 41: 115–118
Wiegand A, Bauer KH, Bonn R, Trenk D, Jähnchen E (1992) Pharmacodynamic and pharmacokinetic evaluation of a new transdermal delivery system with a time-dependent release of glyceryl trinitrate. J Clin Pharmacol 32: 77–84
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vogt, D., Trenk, D., Bonn, R. et al. Pharmacokinetics and haemodynamic effects of ISDN following different dosage forms and routes of administration. Eur J Clin Pharmacol 46, 319–324 (1994). https://doi.org/10.1007/BF00194399
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00194399