Abstract
Since spontaneous reporting of adverse drug reactions depends on the physician's opinion of the relationship between the drug and the adverse event, we compared physicians' opinions with the scores obtained by the causality assessment method used in France. During a 2 month period, all physicians who reported adverse drug reactions (ADRs) to our pharmacovigilance centre expressed their opinions on the causal link by means of visual analogue scales. ADR reports were then assessed with the French causality assessment method by a clinical pharmacologist who was blind to physicians' opinions.
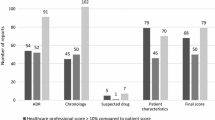
The assessment by both physicians and the standardized method was performed for 75 ADR cases involving 120 drugs. Physicians used a wide range of assessments, with a preponderance of extreme scores, resulting in a U-shaped distribution, while the standardized method gave generally low scores. Scores given by physicians were very high (causality considered very likely or likely) in 60% of cases and very low (causality considered unlikely or dubious/possible) in 32% of cases. Scores obtained using the causality assessment method were low (causality dubious/possible) in 89% of cases and causality considered likely in only 11 cases, essentially in cases with positive rechallenge. Complete agreement occurred in only 6% of cases. Adding complete agreement and minor discrepancies raised the percentage to 49%.
Similar content being viewed by others
References
Stephens MDB (1987) The diagnosis of adverse medical events associated with drug treatment. Adverse Drug React Acute Poisoning Rev 6: 1–35
Bégaud B, Evreux JC, Jouglard J, Lagier G (1985) Unexpected or toxic drug reaction assessment. Therapie 40: 115–118
Meyboom RHB, Royer RJ (1992) Causality classification at pharmacovigilance centres in the European Community. Pharmacoepidemiol Drug Safety 1: 87–97
Bégaud B, Haramburu F, Péré JC, Lorson B, Albin H, Dangoumau J (1982) Le suivi des demandes de renseignements. A propos d'une expérience. Therapie 37: 433–435
Jones JK (1982) Terms used in indicating causal relationship. In: Venulet J, Berneker GG, Ciucci AG (ed) Assessing causes of adverse drug reactions. Academic Press, London, New York, Paris, pp 19–46
Joyce CRB (1982) Identifying causes of disagreement in assessment of causality. In: Venulet J, Berneker GG, Ciucci AG (ed) Assessing causes of adverse drug reactions. Academic Press, London, New York, Paris, pp 95–103
Dangoumau J, Bégaud B, Boisseau A, Albin H (1980) Diagnostics comparés de cliniciens et pharmacologues cliniciens. Nouv Presse Med 9: 1607–1609
Blanc S, Levenberger P, Berger P, Brooke EM, Schelling JL (1979) Judgments of trained observers in adverse drug reactions. Clin Pharmacol Ther 25: 493–498
Hutchinson TA, Flegel KM, Holingkong H, Bloom WS, Kramer MS, Trummer EG (1983) Reasons for disagreement in the standardized assessment of suspected adverse drug reactions. Clin Pharmacol Ther 34: 421–426
Schmidt LG, Dirschedl P, Grohmann R, Scherer J, Wunderlich O, Müller-Oerlinghausen B (1986) Consistency of assessment of adverse drug reactions in psychiatric hospital: a comparison of an algorithmic and an empirical approach. Eur J Clin Pharmacol 30: 199–204
Koch-Weser J, Sellers EM, Zacest R (1977) The ambiguity of adverse drug reactions. Eur J Clin Pharmacol 11: 75–78
Karch FE, Smith CL, Kerzner B, Mazzullo JM, Weintraub M, Lasagna L (1976) Adverse drug reactions — a matter of opinion. Clin Pharmacol Ther 19: 489–492
Haramburu F, Bégaud B, Péré JC (1990) Comparison of 500 spontaneous and 500 published reports of adverse drug reactions. Eur J Clin Pharmacol 39: 207–210
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miremont, G., Haramburu, F., Bégaud, B. et al. Adverse drug reactions: physicians' opinions versus a causality assessment method. Eur J Clin Pharmacol 46, 285–289 (1994). https://doi.org/10.1007/BF00194392
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00194392