Abstract
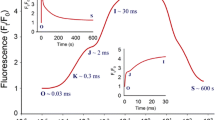
The effects of temperature on the dark relaxation kinetics of nonradiative energy dissipation in photosystem II were compared in lettuce (Lactuca sativa L.) chloroplasts and leaves of Aegialitis annulata R. Br. After high levels of violaxanthin de-epoxidation in the light, Aegialitis leaves showed a marked delay in the dark relaxation of nonradiative dissipation, measured as non-photochemical quenching (NPQ) of photosystem II chlorophyll a fluorescence. Aegialitis leaves also maintained a moderately high adenylate energy charge at low temperatures during and after high-light exposure, presumably because of their limited carbon-fixation capacity. Similarly, dark-sustained NPQ could be induced in lettuce chloroplasts after de-epoxidizing violaxanthin and light-activating the ATP synthase. The duration and extent of dark-sustained NPQ were strongly enhanced by low temperatures in both chloroplasts and leaves. Further, the NPQ sustained at low temperatures was rapidly reversed upon warming. In lettuce chloroplasts, low temperatures sharply decreased the ATP-hydrolysis rate while increasing the duration and extent of the resultant trans-thylakoid proton gradient that elicits the NPQ. This was consistent with a higher degree of energy-coupling, presumably due to reduced proton diffusion through the thylakoid membrane at the lower temperatures. The chloroplast adenylate pool was in equilibrium with the adenylate kinase and therefore both ATP and ADP contributed to reverse coupling. The low-temperature-enhanced NPQ quenched the yields of the dark level (Fo) and the maximal (Fm) fluorescence proportionally in both chloroplasts and leaves. The extent of NPQ in the dark was inversely related to the efficiency of photosystem II, and very similar linear relationships were obtained over a wide temperature range in both chloroplasts and leaves. Likewise, the dark-sustained absorbance changes, caused by violaxanthin de-epoxidation (A508nm) and energy-dependent light scattering (A536nm) were strikingly similar in chloroplasts and leaves. Therefore, we conclude that the dark-sustained, low-temperature-stimulated NPQ in chloroplasts and leaves is apparently directly dependent on lumen acidification and chloroplastic ATP hydrolysis. In leaves, the ATP required for sustained NPQ is evidently provided by oxidative phosphorylation in the mitochondria. The functional significance of this quenching process and implications for measurements of photo-protection versus photodamage in leaves are discussed.
Similar content being viewed by others
Abbreviations
- A:
-
antheraxanthin
- Chl:
-
chlorophyll
- DPS:
-
de-epoxidation state of the xanthophyll cycle, ([Z+A]/[V+A+Z])
- F, F′:
-
steady-state fluorescence in the absence, presence of thylakoid energization
- Fo, F′o :
-
dark fluorescence level in the absence, presence of thylakoid energization
- Fm, F′m :
-
maximal fluorescence in absence, presence of thylakoid energization
- NPQ:
-
nonphotochemical quenching (Fm/F′m)−1
- V:
-
violaxanthin
- Z:
-
zeaxanthin
- NRD:
-
nonradiative dissipation
- PFD:
-
photon flux density
- ɛ:
-
[2ATP+ADP]
- ΔpH:
-
trans-thylakoid proton gradient
- ΔS:
-
ΔpH-dependent light scattering
- ΦPSII :
-
(Fm−F′)/F′m, photon yield of PSII photochemistry at the actual reduction state in the light or dark
- Σ:
-
[ATP+ADP+AMP]
References
Adams WW III, Demmig-Adams B (1995) The xanthophyll cycle and sustained thermal energy dissipation activity in Vinca minor and Euonymous kiautschovicus during the winter. Plant Cell Environ, in press
Adams WW III, Demmig-Adams B (1994) Carotenoid composition and down regulation of photosystem II in three conifer species during the winter. Physiol Plant 92: 451–458
Avron M, Schreiber U (1977) Proton gradients as possible intermediary energy transducers during ATP-driven reverse electron flow in chloroplasts. FEBS Lett 77: 1–6
Ball M (1986) Photosynthesis in mangroves. Wetlands (Australia) 6: 12–22
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25: 173–185
Bilger W, Björkman O (1991) Temperature dependence of violaxanthin de-epoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L. Planta 184: 226–234
Bilger W, Björkman O (1994) Relationship among violaxanthin deepoxidation, thylakoid membrane conformation, and non-photochemical chlorophyll fluorescence in cotton leaves. Planta 193: 238–246
Björkman O (1987) Low-temperature chlorophyll fluorescence in leaves and its relationship to photon yield of photosynthesis in photoinhibition. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Photoinhibition. Elsevier, Amsterdam, pp 123–144
Björkman O, Demmig-Adams B (1994) Regulation of photosynthetic light energy capture, conversion and dissipation in leaves of higher plants. In: Schulze E-D, Caldwell MM (eds) Ecological studies, vol 100, Springer, Berlin, pp 14–47
Björkman O, Demmig B, Andrews TJ (1988) Mangrove photosynthesis: response to high-irradiance stress. Aust J Plant Physiol 15: 43–61
Brugnoli E, Cona A, Lauteri M (1994) Xanthophyll cycle components and capacity for non-radiative energy dissipation in sun and shade leaves of Ligustrum ovalifolium exposed to conditions limiting photosynthesis. Photosynth Res 41: 451–463
Demmig-Adams B, Adams WW III (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43: 599–626
Genty B, Briantais J-M, Baker N (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92
Gilmore AM, Björkman O (1994a) Adenine nucleotides and the xanthophyll cycle in leaves I.Effects of CO2 and temperature limited photosynthesis on the adenylate energy charge and violaxanthin de-epoxidation. Planta 192: 526–536
Gilmore AM, Björkman O (1994b) Adenine nucleotides and the xanthophyll cycle in leaves II.Comparison of the effects of CO2 and temperature limited photosynthesis on photosystem II fluorescence quenching, the adenylate energy charge and violaxanthin de-epoxidation in cotton. Planta 192: 537–544
Gilmore AM, Yamamoto O (1991) Resolution of lutein and zeaxanthin using a nonendcapped, lightly carbon-loaded C-18 high-performance liquid chromatographic column. J Chromatogr 543: 137–145
Gilmore AM, Yamamoto HY (1992a) Dark induction of zeaxanthin-dependent nonphotochemical fluorescence quenching mediated by ATP. Proc Natl Acad Sci USA 89: 1899–1903
Gilmore AM, Yamamoto HY (1992b) Zeaxanthin-dependent quenching of the variable fluorescence arising from ATP-induced reverse electron flow. In: Murata, N (ed) Research in photosynthesis, vol I. Kluwer, Dordrecht, pp 255–258
Gilmore AM, Yamamoto HY (1993) Linear models relating xanthophylls and lumen acidity to non-photochemical fluorescence quenching. Evidence that antheraxanthin explains zeaxanthin-independent quenching. Photosynth Res 35: 67–78
Gong H, Nilsen S (1989) Effect of temperature on photoinhibition of photosynthesis, recovery, and turnover of the 32 kD chloroplast protein in Lemna gibba. J Plant Physiol 135: 9–14
Hager A (1969) Lichtbedingte pH-Erniedrigung in einem Chloroplasten-Kompartiment als Ursache der enzymatischen Violaxanthin — Zeaxanthin-Umwandlung; Beziehungen zur Photophosphorylierung. Planta 89: 224–243
Hanning I, Heldt HW (1993) On the function of mitochondrial metabolism during photosynthesis in spinach (Spinacia oleracea L.) leaves. Plant Physiol 103: 1147–1154
Kramer DM, Wise RR, Frederick JR, Alm DM, Hesketh JD, Ort DR, Crofts AR (1990) Regulation of coupling factor activity in field grown sunflower: A Redox model relating coupling factor activity to the activities of other thioredoxin-dependent chloroplast enzymes. Photosynth Res 26: 213–222
Kyle DJ (1987) The biochemical basis for photoinhibition of photosystem II. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Photoinhibition. Elsevier, Amsterdam, pp 197–226
Mills JD, Mitchell P (1982) Modulation of coupling factor ATPase activity in intact chloroplasts. Reversal of thiol modulation in the dark. Biochim Biophys Acta 679: 75–83
Mohanty N, Gilmore AM, Yamamoto HY (1995) Mechanism of non-photochemical chlorophyll fluorescence quenching: II. Resolution of rapidly reversible absorbance changes at 530 nm and fluorescence quenching by the effects of antimycin, dibucaine and cation exchanger A23187. Aust J Plant Physiol 22: 239–247
Osmond CB (1994) What is photoinhibition? Some insights from comparisons of shade and sun plant. In: Baker NR, Bowyer JR (eds) Photoinhibition of photosynthesis. Bios, Oxford, pp 1–24
Petrack B, Lipman F (1961) Photophosphorylation and photohyd-rolisis in cell-free preparations of blue-green alga. In: Mc Elroy WD, Glass B (eds) Light and life. John Hopkins Press, Baltimore, pp 621–630
Ruban AV, Rees D, Noctor GD, Young A, Horton P (1991) Long wavelength chlorophyll species are associated with amplification of high-energy-state excitation quenching. Biochim Biophys Acta 1059: 355–360
Schatz GH, Brock H, Holzwarth AR (1988) A kinetic and energetic model for the primary processes in photosystem II. Biophys J 54: 397–405
Schreiber U, Avron M (1979) Properties of ATP-driven reverse electron flow in chloroplasts. Biochim Biophys Acta 546: 436–447
Schreiber U (1980) Light-activated ATPase and ATP-driven reverse electron transport in intact chloroplasts. FEBS Lett 122: 121–124
van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Yamamoto HY (1962) Studies on the light and dark interconversions of leaf xanthophylls. Arch Biochem Biophys 97: 168–173
Yamamoto HY, Kamite L (1972) The effects of dithiothreitol on violaxanthin de-epoxidation and absorbance changes in the 500-nm region. Biochim Biophys Acta 267: 538–543
Author information
Authors and Affiliations
Additional information
We thank Connie Shih for skillful assistance in growing plants and for conducting HPLC analyses. Support from an NSF/USDA/DOE postdoctoral training grant to A.G. is gratefully acknowledged. A.G. also wishes to thank Prof. Govindjee for valuable discussions. C.I.W.-D.P.B. Publication No. 1197.
Rights and permissions
About this article
Cite this article
Gilmore, A.M., Björkman, O. Temperature-sensitive coupling and uncoupling of ATPase-mediated, nonradiative energy dissipation: Similarities between chloroplasts and leaves. Planta 197, 646–654 (1995). https://doi.org/10.1007/BF00191573
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00191573