Abstract
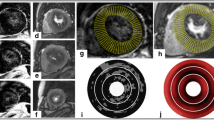
The aim of this study was to test the hypothesis that increased mechanical stress at the lateral borders of the area at risk may render this area more susceptible to ischaemia/reperfusion injury in the absence of collateral flow. The spatial distribution of myocardial necrosis within the territory of a transiently occluded left anterior descending coronary artery was investigated in 31 porcine hearts submitted to 48 min of coronary occlusion and 6 h of reperfusion. Immediately before excising the heart, the left anterior descending coronary artery was re-occluded and 10% fluorescein was injected in the left atrium. The area at risk was imaged by ultraviolet illumination of the myocardial slices, and the area of necrosis by incubation in triphenyltetrazolium chloride. The area at risk was divided in four sectors and an index of eccentricity was calculated as the percent of the area of necrosis located in the two lateral sectors of the area at risk. The area of contraction band necrosis was measured in whole heart histological sections. Infarcts were generally small, and were composed almost exclusively of contraction band necrosis. There was a good correlation between the extent of the area of contraction band necrosis and infarct size (r=0.831, P<0.0005). The area of necrosis had a patchy appearance and was predominantly distributed along the lateral borders of the area at risk. This eccentric distribution was more prominent in smaller infarcts, and the eccentricity index was inversely correlated with infarct size (r=−0.471, P=0.007), suggesting that contraction band necrosis occurres first at the interface between control and reperfused myocardium in this model. These results are in agreement with a prominet role of mechanical factors in the genesis of myocardial necrosis during transient coronary occlusion.
Similar content being viewed by others
References
Batts K, Ackerman D, Edwards W (1990) Postinfarction rupture of the left ventricular free wall: clinicopathologic correlates in 100 consecutive autopy cases. Hum Pathol 21:530–535
Cinca J, Worner F, Carreño A, Coronel R, Soldevilla A, Pérez-Villa F, Janse MJ, Soler-Soler J (1992) T-Q, S-T segment mapping and hyperemia in reperfused pig heart with ischemic preconditioning. Am J Physiol 263:H1732-H1738
Coronel R, Fiolet JWT, Wilms-Shopman FJG, Opthof T, Schaapherder AFM, Janse MJ (1989) Distribution of extracellular potassium and electrophysiologic changes during two-stage coronary ligation in the isolated, perfused canine heart. Circulation 80:165–177
Fishbein MC, Maclean D, Maroko PR (1978) The histopathologic evolution of myocardial infarction. Chest 73:843–849
Frank JS, Brady AJ, Fransworth S, Mottino G (1986) Ultrastructure and function of isolated myocytes after calcium depletion and repletion. Am J Physiol 250:H265-H275
Fujiwara H, Ashraf M, Sato S, Millard RW (1982) Transmural cellular damage and blood flow distribution in early ischemia in pig hearts. Circ Res 51:683–693
Ganote C, Armstrong S (1993) Ischaemia and the myocyte cytoskeleton: review and speculation. Cardiovasc Res 27:1387–1403
Hearse J, Humphrey SM, Chain EB (1973) Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol 5:395–407
García-Dorado D, Théroux P, Desco M, Solares J, Elizaga J, Fernandez-Avilés F, Alonso J, Soriano J: Cell-to-cell interaction (1989) A mechanism to explain wave-front progression of myocardial necrosis. Am J Physiol 256:H1266-H1273
Garcia-Dorado D, Théroux P, Solares J, Alonso J, Fernandez-Avilés F, Elizaga J, Soriano J, Botas J, Muñoz R (1990) Determinants of hemorrhagic infarcts. Histologic observations from experiments involving coronary occlusion, coronary reperfusion and reocclusion. Am J Pathol 173:301–311
Garcia-Dorado D, Théroux P, Duran JM, Solares J, Alonso J, Sanz E, Muñoz R, Elizaga J, Botas J, Fernandez-Avilés F, Soriano J, Esteban E (1992) Selective inhibition of the contractile apparatus. A new approach to modification of infarct size, infarct composition, and infarct geometry during coronary artery occlusion and reperfusion. Circulation 85:1160–1174
Garcia-Dorado D, Oliveras J, Gili J, Sanz E, Pérez-Villa F, Barrabés J, Carreras MJ, Solares J, Soler Soler J (1993) Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res 27:1462–1469
Griffith GCG, Hegde B, Oblath RW (1961) Factors in myocardial rupture. An analysis of two hundred and four cases at Los Angeles County Hospital between 1924 and 1955. Am J Cardiol 8:792–798
Litt MR, Jerremy RW, Wisman HS, Winkestein JA, Becker LC (1989) Neutrophil depletion limited to reperfusion reduces myocardial infarct size after 90 minutes of ischemia. Evidence for neutrophil mediated reperfusion injury. Circulation 80:1816–1827
Miyazaki S, Fujiwara H, Onodera T, Kihara Y, Matsuda M, Wu D-J, Nakamura Y, Kumada T, Sasayama S, Kawai C, Hamashima Y (1987) Quantitative analysis of contraction band and coagulation necrosis after ischemia and reperfusion in the porcine heart. Circulation 75:1074–1082
O'Brien P, Bosnjak Z, Warltier DC (1993) Recovery of contractile function of postischemic, reperfused myocardium. Influence of 2,3-Butanedione-2-monoxime. J Cardiovasc Pharmacol 21:693–700
Oliva PB, Hammill SC, Edwards WD (1993) Cardiac rupture: a clinically predictable complication of acute myocardial infarction. Report of 70 cases with clinicopathologic correlations. J Am Coll Cardiol 22:720–726
Reimer KA, Jennings RB (1979) The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest 40:633–644
Reimer KA, Jennings RB (1992) Myocardial ischemia, hypoxia, and infarction. In: Fozzard HA, Haber E, Jennings RB, Katz A, Morgan HE (eds) The heart and cardiovascular scientific fundations. Raven Press, New York, p 1886
Rousseau G, Hebert D, Libersan D, Khadil A, G SJ, Latour JG (1993) Importance of platelets in myocardial injury after reperfusion in the pressence of residual coronary stenosis in dogs. Am Heart J 125:1553–1563
Sabbah HN, Stein PD (1982) Effect of acute regional ischemia on pressure in the subepicardium and subendocardium. Am J Physiol 242:H240-H244
Savage RM, Wagner GS, Ideker RE, Podolsky SA, Hackel DB (1977) Correlation of postmortem anatomic findings with electrocardiographic changes in patients with myocardial infarction. Retrospective study of patients with typical anterior and posterior infarcts. Circulation 55:279–285
Siegmund B, Klietz T, Schwartz P, Piper HM (1991) Temporary contractile blockade prevents hypercontracture in anoxic-reoxygenated cardiomyocytes. Am J Physiol 260:H426-H435
Siegmund B, Koop A, Klietz T, Schwartz P, Piper HM (1990) Sarcolemmal integrity and metabolic competence of cardiomyocytes under anoxia-reoxygenation. Am J Physiol 258: H285-H291
Steenbergen C, Hill ML, Jennings RB (1985) Volume regulation and plasma membrane injury in aerobic, anaerobic, and ischemic myocardium in vitro. Effects of osmotic cell swelling on plasma membrane integrity. Circ Res 57:864–875
Steenbergen C, Hill ML, Jennings RB (1987) Cytoskeletal damage during myocardial ischemia: changes in vinculin immunofluorescence staining during total in vitro ischemia in canine heart. Circ Res 60:478–486
Author information
Authors and Affiliations
Additional information
Partially supported by Grants 92/0443 from the Fondo de Investigaciones Sanitarias de la Seguridad Social, CRHG 07-92/79 from the Institut Català de la Salut, and Concerted Action BMH1-CT92/1501, BIOMED I Program from the European Union.
Rights and permissions
About this article
Cite this article
Solares, J., Garcia-Dorado, D., Oliveras, J. et al. Contraction band necrosis at the lateral borders of the area at risk in reperfused infarcts. Vichows Archiv A Pathol Anat 426, 393–399 (1995). https://doi.org/10.1007/BF00191349
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00191349